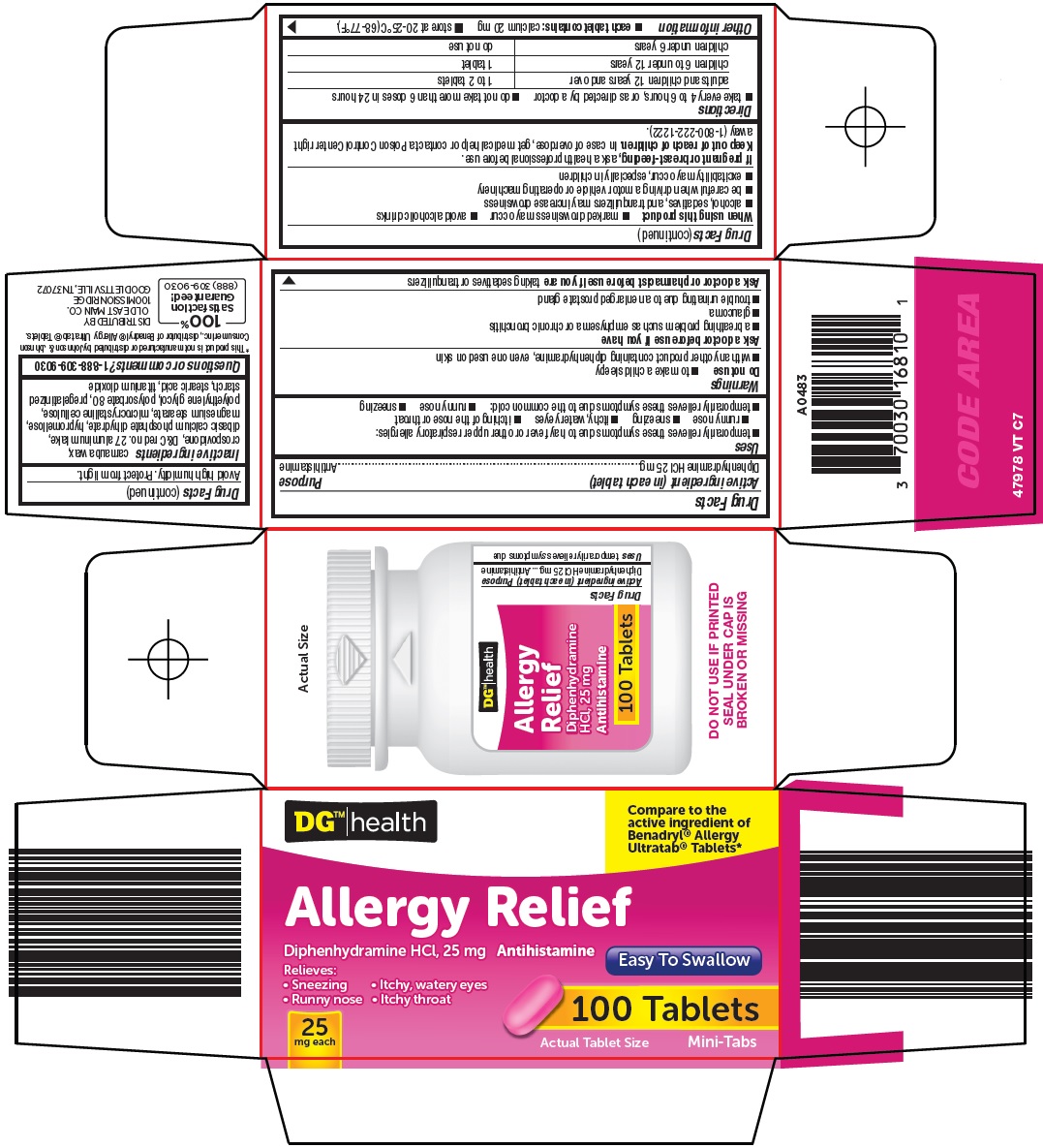
Uses
- •
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- itchy nose or throat
- •
- temporarily relieves these symptoms of the common cold:
- •
- runny nose
- •
- sneezing
Warnings
Do not use
- •
- to make a child sleepy
- •
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- glaucoma
- •
- trouble urinating due to an enlarged prostate gland
Directions
- •
- take every 4 to 6 hours, or as directed by a doctor
- •
- do not take more than 6 doses in 24 hours
|
adults and children 12 years and over |
1 to 2 tablets |
|
children 6 to under 12 years |
1 tablet |
|
children under 6 years |
do not use |
Other information
- •
- each tablet contains: calcium 20 mg
- •
- store at 20-25°C (68-77°F). Avoid high humidity. Protect from light.
Active ingredients
carnauba wax, crospovidone, D&C red no. 27 aluminum lake, dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, stearic acid, titanium dioxide
Principal Display Panel
DG™ Health
Compare to the active ingredient of Benadryl® Allergy Ultratab® Tablets
Allergy Relief
Diphenhydramine HCl, 25 mg
Antihistamine
Easy To Swallow
Relieves:
● Sneezing
● Itchy, watery eyes
● Runny nose
● Itchy throat
25 mg each
100 Tablets
Actual Tablet Size
Mini-Tabs