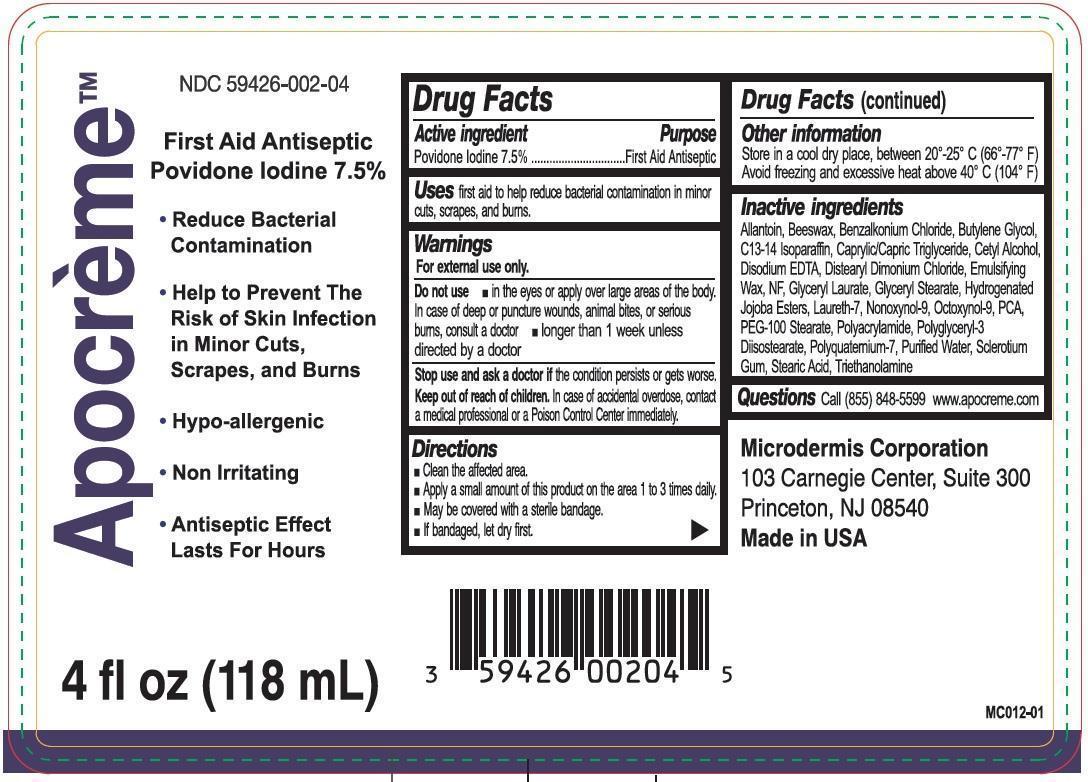
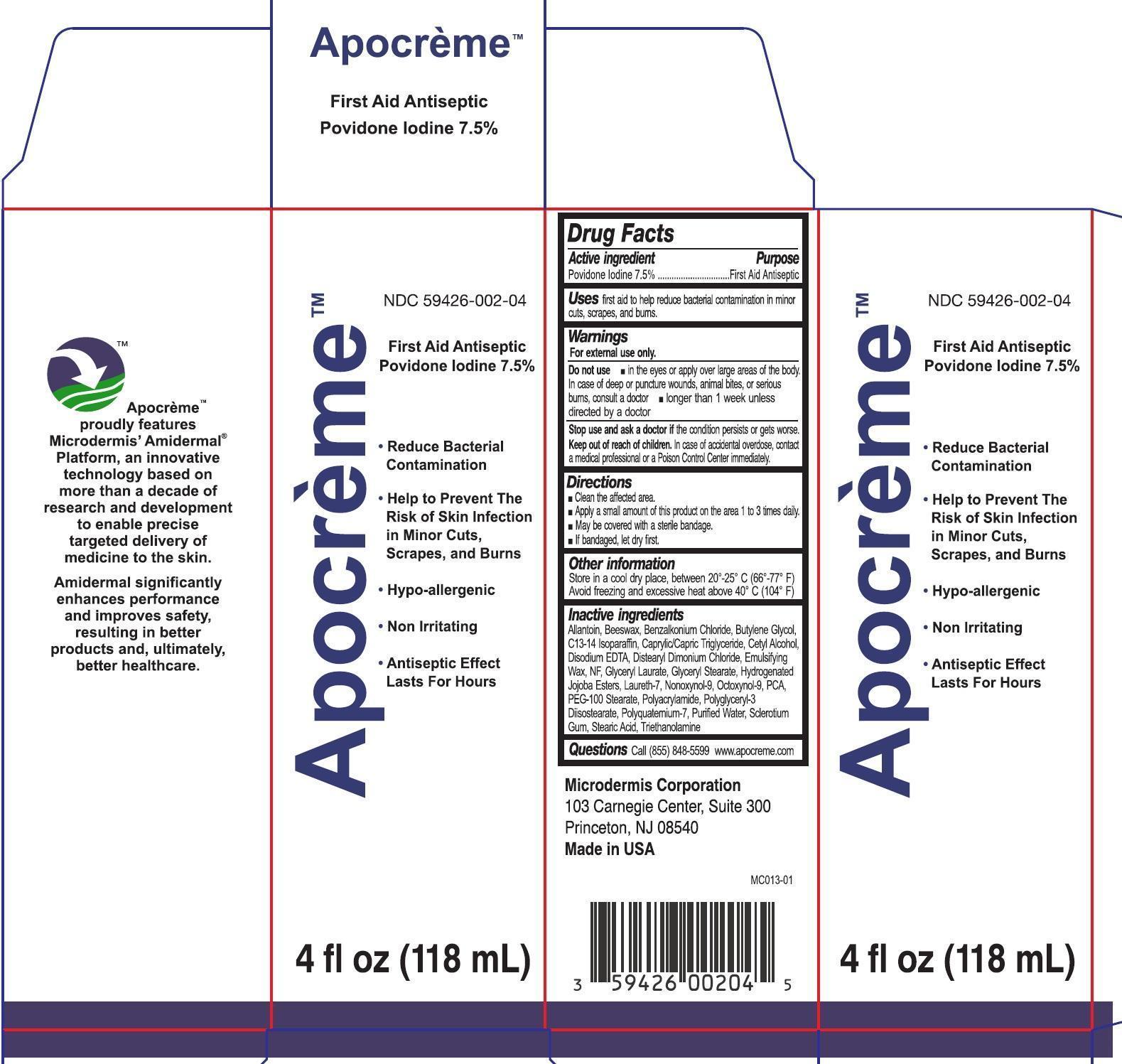
APOCREME- povidone iodine emulsion
Microdermis Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Povidone Iodine 7.5%
Purpose
First Aid Antiseptic
Uses
First aid to help reduce bacterial contamination in minor cuts, scrapes, and burns
Warnings
For external use only.
Do not use
- In the eyes or apply over large areas of the body. In case of deep or puncture wounds, animals bites, or serious burns, consult a doctor
- Longer than 1 week unless directed by a doctor
Stop use and ask a doctor
If the condition persists or gets worse.
Keep out of reach of children.
In case of accidental overdose, contact a medical professional or a Poison Control Center immediately.
Directions
- Clean the affected area.
- Apply a small amount of this product on the area 1 to 3 times daily.
- May be coveres with a sterile bandage.
- If bandaged, let dry first.
Other information
Store in a cool dry place, between 20o-25o C (66o -77o F)
Avoid freezing and excessive heat above 40o C (104o F)
Inactive ingredients
Allantoin, Beeswax, Benzalkonium Chloride, Butylene Glycol, C13-14 Isoparaffin, Caprylic/Capric Triglyceride, Cetyl Alcohol, Disodium EDTA, Distearyl Dimonium Chloride, Emulsifying Wax, NF, Glyceryl Laurate, Glyceryl Stearate, Hydeogenated Jojoba Esters, Laureth-7, Nonoxynol-9, Octoxynol-9, PCA, PEG-100 Stearate, Polyacrylamide, Polyglyceryl-3 Diisostearate, Polyquatemium-7, Purified Water, Sclerotium Gum, Stearic Acid, Triethanolamine
Questions
Call (855) 848-5599 www.apocreme.com
Product Label