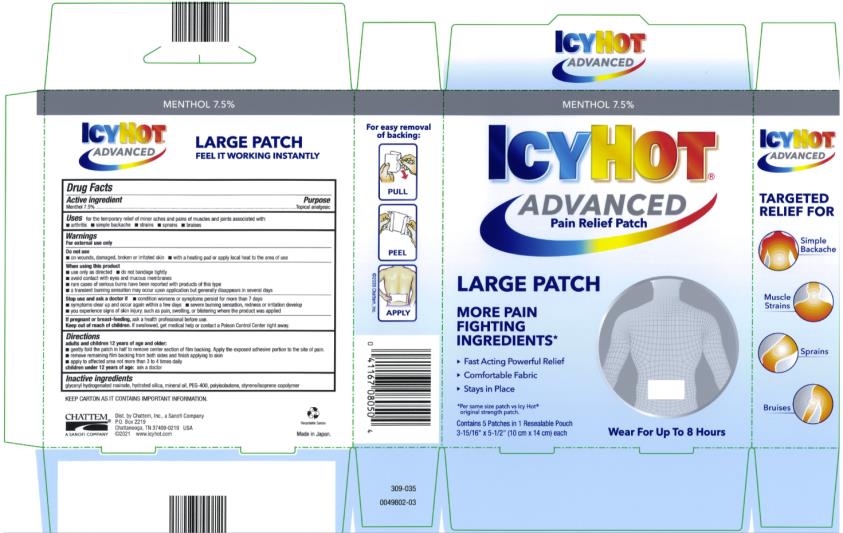
Uses
for the temporary relief of minor aches and pains of muscles and joints associated with:
■ arthritis ■ simple backache ■ strains ■ sprains ■ bruises
Warnings
For external use only
Do not use
■ on wounds, damaged, broken or irritated skin
■ with a heating pad or apply local heat to the area of use
When using this product
■ use only as directed
■ do not bandage tightly
■ avoid contact with eyes and mucous membranes
■ rare cases of serious burns have been reported with products of this type
■ a transient burning sensation may occur upon application but generally disappears in several days
Stop use and ask a doctor if
■ condition worsens or symptoms persist for more than 7 days
■ symptoms clear up and occur again within a few days
■ severe burning sensation, redness or irritation develop
■ you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
Directions
adults and children 12 years of age and older:
■ gently fold the patch in half to remove center section of film backing. Apply the exposed adhesive portion to the site of pain.
■ remove remaining film backing from both sides and finish applying to skin
■ apply to affected area not more than 3 to 4 times daily
children under 12 years of age: consult a doctor