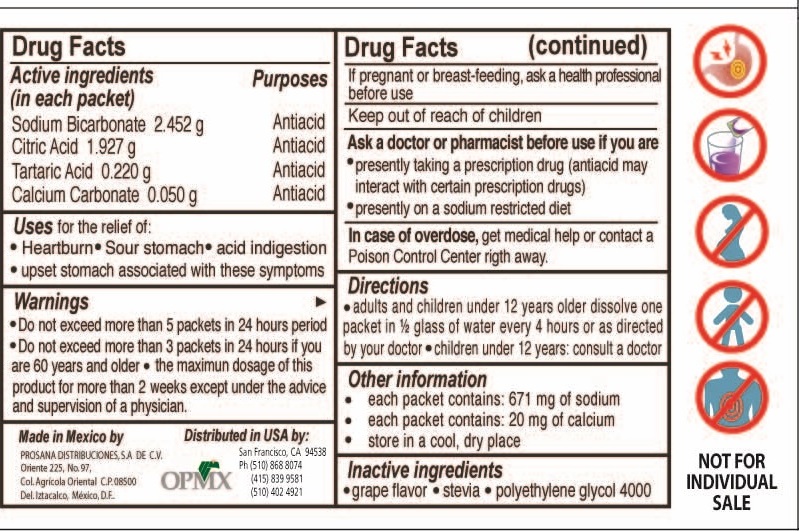
BICARUVAS- antacid powder, for solution
PROSANA DISTRIBUCIONES, S.A. DE C.V.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Bicaruvas
Double Action
BICARUVAS
.Sodium Bicarbonate
.Calcium Carbonate
Antiacid
Effervescent Powder
8 packets .17 oz (5g) ea.

Directions
.Adult and children 12 years older dissolve one packet in 1/2 glass of water every 4 hours or as directed by your doctor.
Children under 12 years, consult a doctor
Principal Display
Please see box.jpg

Active ingredients
Active Ingredients (in each packet) Purpose
Sodium Bicarbonate 2.452g antacid
Citric Acid 1.927 g antiacid
Tartaric Acid 0.220g antiacid
Calcium Carbonate .05 g antiacid

Uses
Uses for the relief of:
Heartburn . Sour stomach . upset stomach associated with these symptoms

Warnings
Warnings Do not exceed more than 5 packets in a 24 hour period
Do not exceed more than 3 packets in 24 hours if you are 60 years or older
the maximum dosage for more than two weeks except under the advise and supervision of a physician

Pregnant
If pregnant or breat feeding, ask a helth professional before use

Overdose
In case of Overdose get medical help or contact a Poison Control Center right away.
Inactive
Inactive Ingredients
grape flavor. stevia. polyethilene glycol 4000
Other Information
Other Information
each packet contains 671 mg of sodium
each packet contains 20 mg of calcium
store in a dry cool place
children
Keep out of reach of children

PROSANA DISTRIBUCIONES, S.A. DE C.V.




