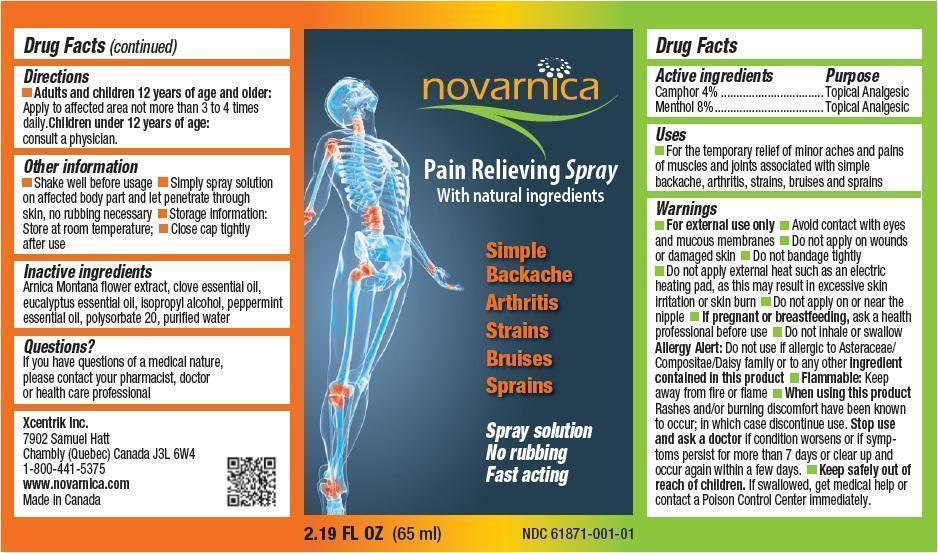
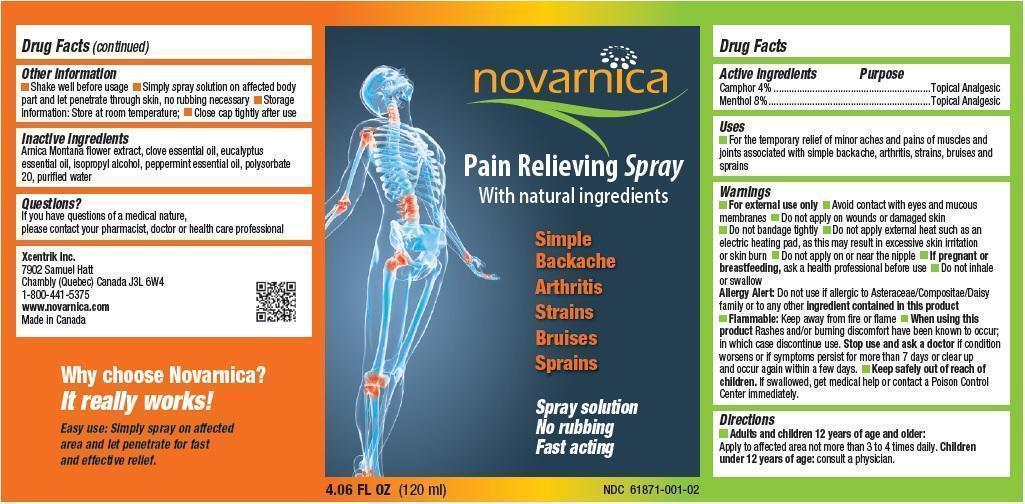
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises and sprains
Warnings
- For external use only
- Avoid contact with eyes and mucous membranes
- Do not apply on wounds or damaged skin
- Do not bandage tightly
- Do not apply external heat such as an electric heating pad, as this may result in excessive skin irritation or skin burn
- Do not apply on or near the nipple
- If pregnant or breastfeeding, ask a health professional before use
- Do not inhale or swallow
- Allergy Alert: Do not use if allergic to Asteraceae/Compositae/Daisy family or to any other ingredient contained in this product
- Flammable: Keep away from fire or flame
-
When using this product Rashes and/or burning discomfort have been known to occur; in which case discontinue use. Stop use and ask a doctor if condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days.
Directions
- Adults and children 12 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- Children under 12 years of age: consult a physician.
Other information
- Shake well before usage
- Simply spray solution on affected body part and let penetrate through skin, no rubbing necessary
- Storage information: Store at room temperature;
- Close cap tightly after use
Inactive ingredients
Arnica Montana flower extract, clove essential oil, eucalyptus essential oil, isopropyl alcohol, peppermint essential oil, polysorbate 20, purified water
Questions?
If you have questions of a medical nature, please contact your pharmacist, doctor or health care professional
Xcentrik Inc.
7902 Samuel Hatt
Chambly (Quebec) Canada J3L 6W4
1-800-441-5375
www.novarnica.com
Made in Canada