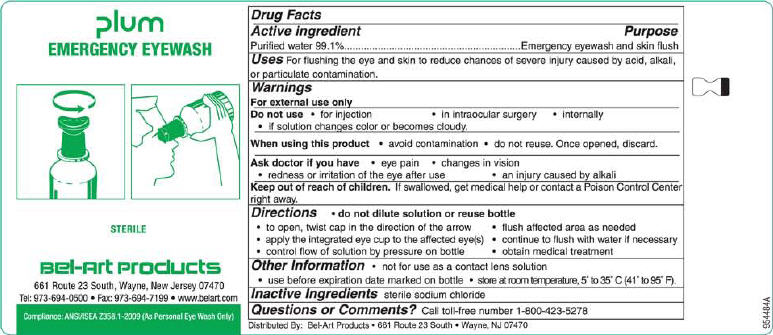
PLUM EMERGENCY EYEWASH - water liquid
Bel-Art Products
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Purified water 99.1%
Purpose
Emergency eyewash and skin flush
Uses
For flushing the eye and skin to reduce chances of severe injury caused by acid, alkali, or particulate contamination.
Warnings
For external use only
Do not use • for injection • in intraocular surgery • internally
• if solution changes color or becomes cloudy.
When using this product • avoid contamination, do not touch tip of container to any surface
• do not reuse. Once opened, discard.
Ask doctor if you have • eye pain • changes in vision
• redness or irritation of the eye after use • an injury caused by alkali
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center
right away.
Directions • do not dilute solution or reuse bottle
• hold container a few inches above the eye or skin
• control flow of solution by pressure on bottle • flush affected area as needed
• continue to flush with water if necessary • obtain medical treatment
Other Information • not for use as a contact lens solution • twist cap to open
• use before expiration date marked on bottle • store at room temperature, 5° to 35° C (41° to 95° F).
Inactive Ingredients sterile sodium chloride
Questions or Comments? Call toll-free number 1-800-423-5278.
Plum
EMERGENCY EYEWASH
NDC 67691-100-02
STERILE
200 ml (6.76 fl oz)
Bel-Art Products
661 Route 23 South, Wayne, New Jersey 07470
Tel: 973-694-0500 • Fax: 973-694-7199 • www.belart.com
Compliance: ANSI Z358.1-2004 (As Personal Eye Wash Only)
Plum Eyewash Saline Label.jpg
Plum Eyewash Saline Label.jpg
