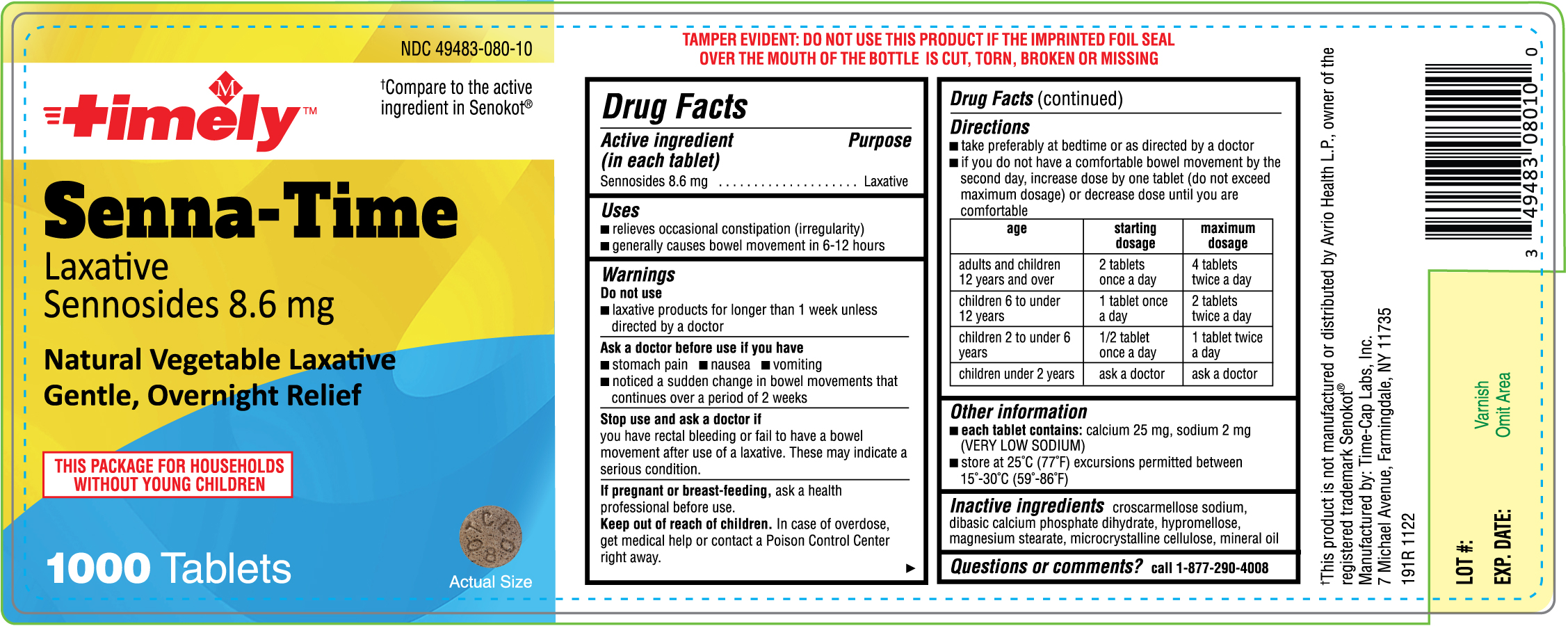
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel movements that continues over a period of 2 weeks
Stop use and ask a doctor if
you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- take preferably at bedtime or as directed by a doctor
- if you do not have a comfortable bowel movement by the second day, increase dose by one tablet (do not exceed maximum dosage) or decrease dose until you are comfortable
age
starting dosage maximum dosage adults and children 12 years and over 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor
OTHER INFORMATION
Other information
- each tablet contains: calcium 25 mg,sodium 2 mg (VERY LOW SODIUM)
- store at 25°C (77°F) excursions permitted between 15°-30°C (59°-86°F)