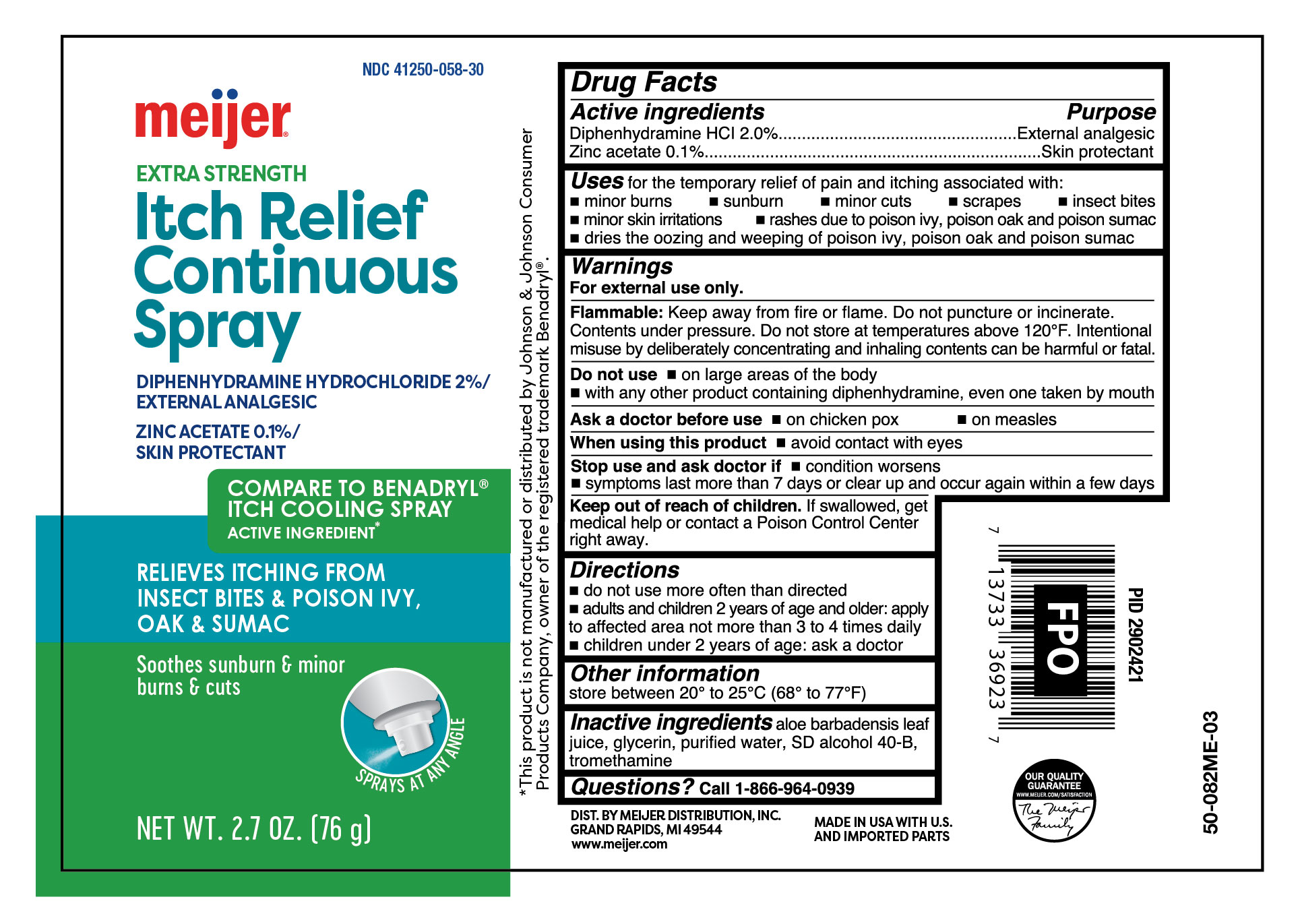
Active ingredients
Diphenhydramine HCl 2.0%,
Zinc acetate 0.1%
Purpose
- External analgesic
- Skin protectant
Uses
for the temporary relief of pain and itching associated with:
- minor burns
- sunburn
- minor cuts
- scrapes
- insect bites
- minor skin irritations
- rashes due to poison ivy, poison oak and poison sumac
- dries the oozing and weeping of poison ivy, poison oak and poison sumac
Warnings
For external use only.
Flammable:
Keep away from fire or flame. Do not puncture or incinerate. Contents under pressure. Do not store at temperatures above 120ºF. Intentional misuse by deliberately concentrating and inhaling contents can be harmful or fatal.
Do not use
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
Ask a doctor before use
- on chicken pox
- on measles
Stop use and ask doctor if
- conditions worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- do not use more often than directed
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Other information
store between 20° to 25°C (68° to 77°F)
Inactive ingredients
aloe barbadensis leaf juice, glycerin, purified water, SD alcohol 40-B, tromethamine
Questions?
call 1-866-964-0939
Principal Display Panel
meijer
CONTINUOUS
itch relief
spray
Diphenhydramine HCl
Zinc Acetate
External Analgesic
Skin Protectant
EXTRA STRENGTH
Relieves Itching from insect bites
and poison ivy, oak & sumac
Soothes sunburn and
minor burns & cuts
NET WT 2.7 OZ (76 g)