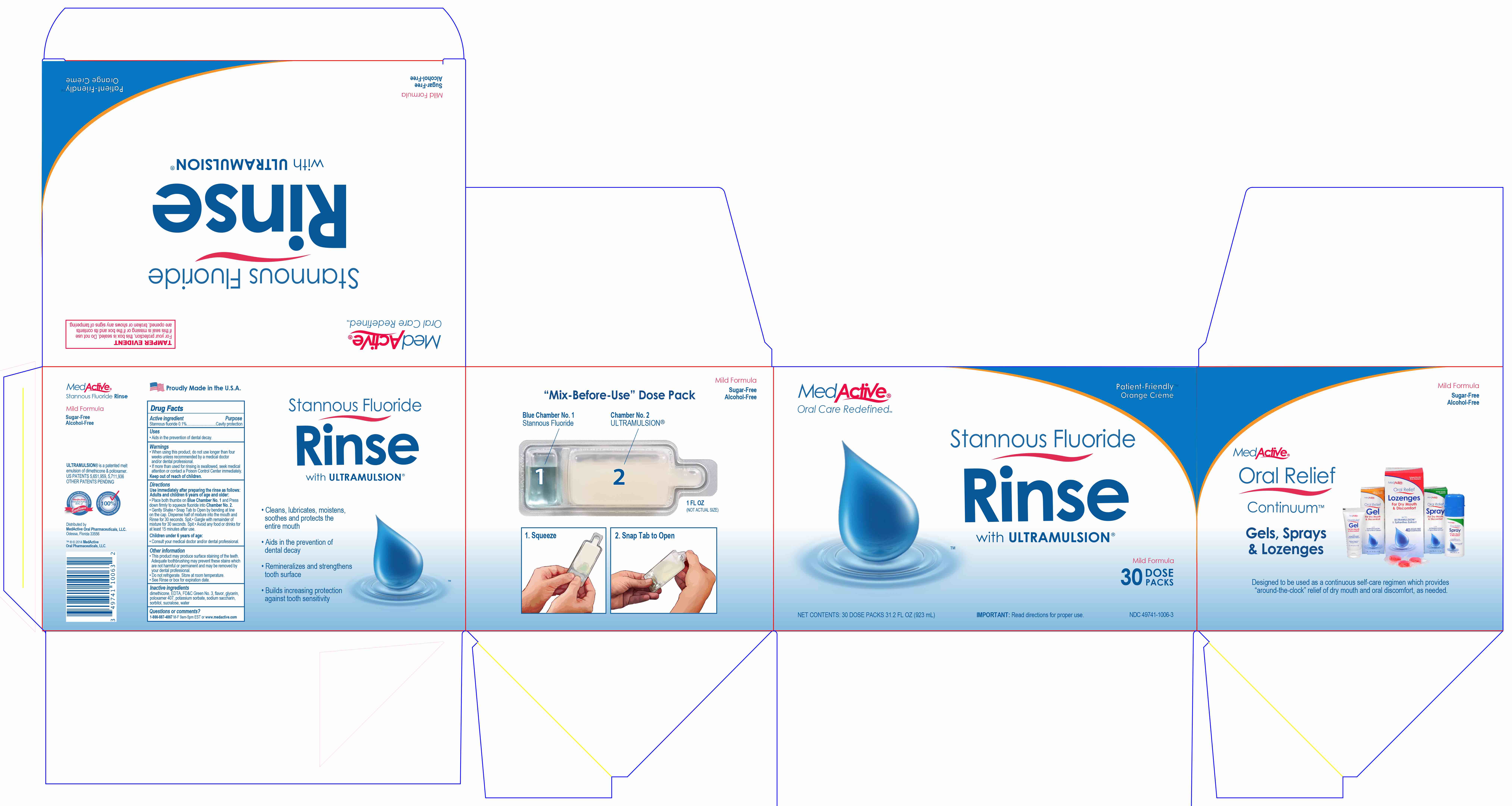
Warnings
- When using this product, do not use longer than four weeks unless recommended by a medical doctor and/or dental professional.
- If more than used for rinsing is swallowed, seek medical attention or contact a Poison Control Center immediately.
Directions
Use immediately after preparing the rinse as follows:
Adults and children 6 years or age and older:
- Place both thumbs on Blue Chamber No. 1 and Press down firmly to squeeze fluoride into Chamber No. 2.
- Gently Shake.
- Snap Tab to Open by bending at line on the cap. Dispense half of mixture into the mouth and Rinse for 30 seconds. Spit.
- Gargle with remainder of mixture for 30 seconds. Spit.
- Avoid any food or drinks for at least 15 minutes after use.
Children under 6 years of age:
- Consult your medical doctor and/or dental professional.
Other Information
- This product may produce surface staining of the teeth. Adequate toothbrushing may prevent these stains which are not harmful or permanent and may be removed by your dental professional.
- Do not refrigerate. Store at room temperature.
- See Rinse or box for expiration date.
Inactive Ingredients
dimethocone, EDTA, FD&C Green No. 3, flavor, glycerin, poloxamer 407, potassium sorbate, sodium saccharin, sorbital, sucralose, water
 MedActive
MedActive
 MedActive
MedActive