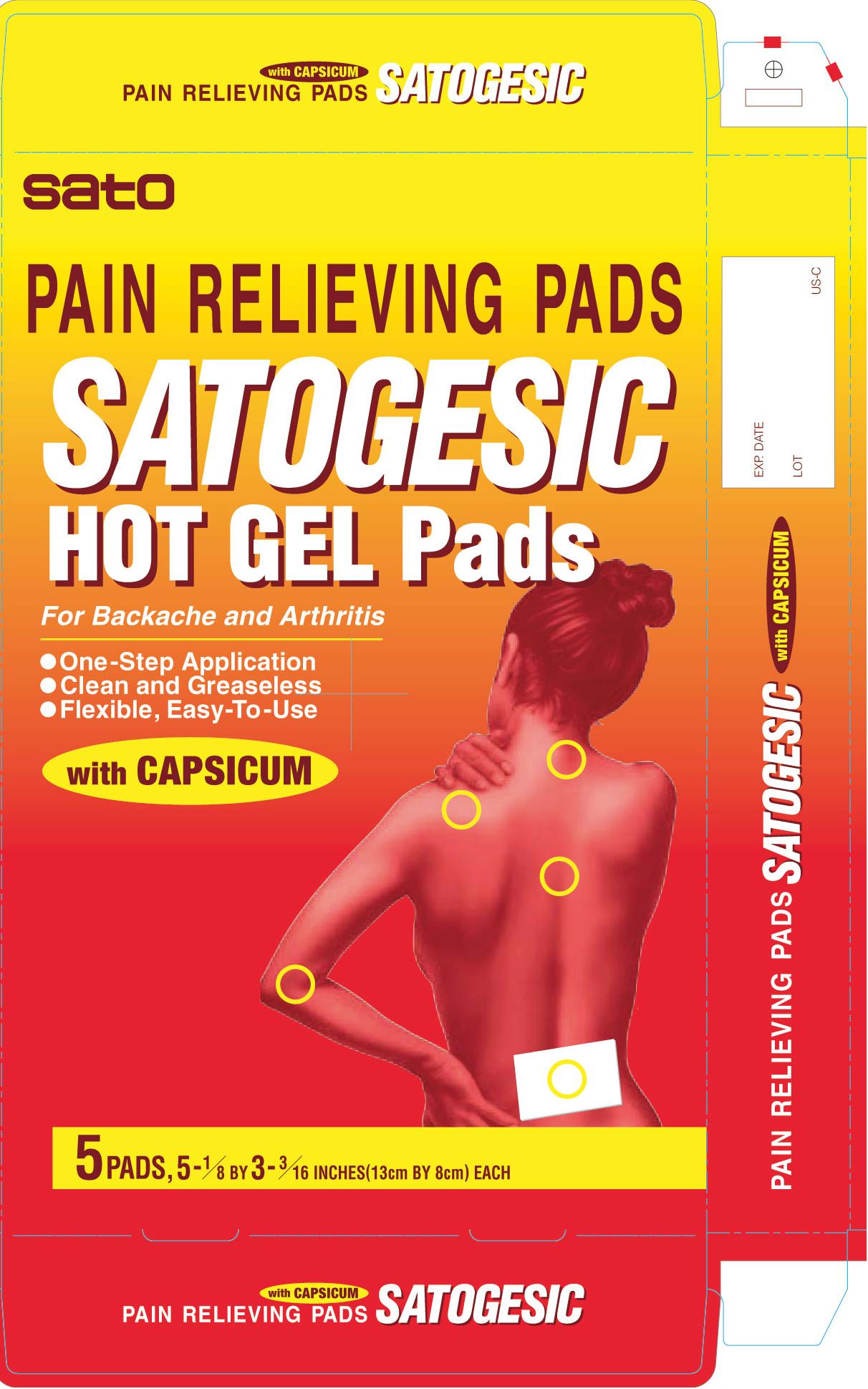
Uses temporary relieves minor aches and pains of muscles and joints associated with
■ simple backache ■ arthritis ■ strains ■ bruises ■ sprains
Warnings
For external use only
Do not use
■ on wounds, or on damaged, or irritated skin
■ on children under 12 years with arthritis-like conditions unless directed by a doctor
When using this product
■ avoid contact with eyes or mucous membranes ■ do not cover with any additional wrap
Other information
■ store unused pads in the pouch ■ reseal the pouch after opening and keep tightly closed
Directions
■ Adults and children 2 years of age and over:
■ strip off the polyethylene film and place adhesive pad over affected area
■ pad may be cut with scissors for small areas such as fingers
■ change pad 1 or 2 times a day
■ apply to affected area not more than 3 or 4 times daily
■ Children under 2 years of age: do not use, consult a doctor
Inactive ingredients
Butylparaben, carbomer, carboxymethylcellulose sodium, dried aluminum hydroxide gel, edetate disodium, glycerin, malic acid, methacrylic acid and n-butyl acrylate copolymer, methylparaben, partially neutralized polyacrylate, polyethylene glycol 3000 hydrogenated castor oil, polyvinyl alcohol, purified water, sorbitol, titanium dioxide