INDICATIONS:
For use as a diagnostic agent when superficial corneal or conjunctival tissue change is suspected.
DIRECTIONS FOR USE:
For best viewing and patient comfort, the Lissamine Green impregnated tip should be moistened with one or two drops of sterile irrigating or saline solution before application. Apply moistened tip to conjunctiva or fornix as required. It is recommended that the patient blink several times after application.
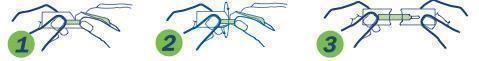
1. Hold package in middle and tear down toward sterile strip
2. Turn over and tear down from other side, taking care not to tear the sterile strip.
3. Grasp each end of package firmly as shown, without touching the sterile strip, and pull apart.
NOTE:
Not to be used with soft contact lenses. For external use only. Keep out of the reach of childred.
Manufactured by:
Contacare Ophthalmics & Diagnositics (EOU)
Block No. 310, Village Sim of Dabhasa, Taluka: Padra,
Dist.: Vadodara - 391 440, Gujarat, India.
Mfg. Lic. No. G/1197
Manufactured for, and distributed by:
HUB Pharmaceuticals, LLC
Rancho Cucamonga, CA 91730
www.hubrx.com
European Representative:
MDT J.Zych, A. Budyn Sp j.
ul. Skosna 12A, 30-383 Krakow, Poland

