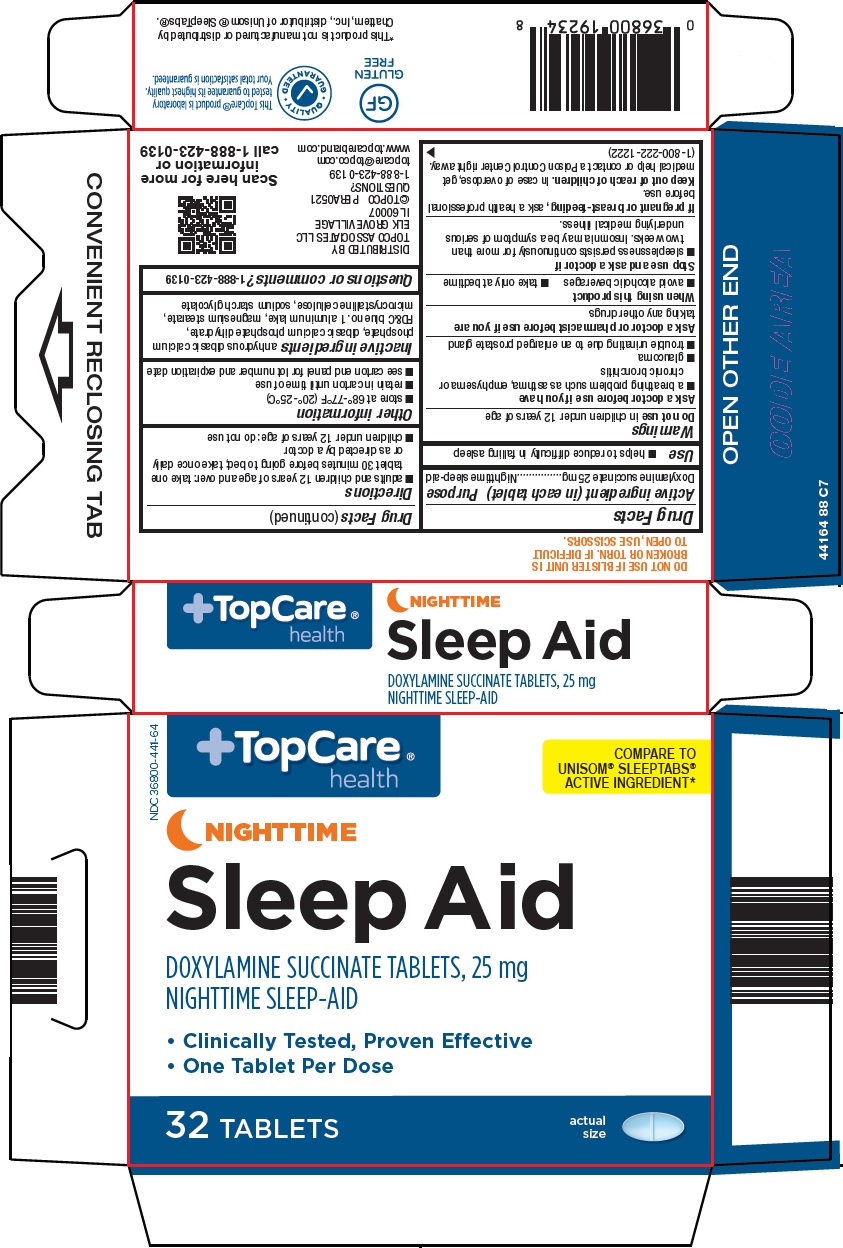
Active ingredient (in each tablet)
Doxylamine succinate 25 mg
Purpose
Nighttime sleep-aid
Use
- •
- helps to reduce difficulty in falling asleep
Warnings
Do not use
in children under 12 years of age
Ask a doctor before use if you have
- •
- a breathing problem such as asthma, emphysema or chronic bronchitis
- •
- glaucoma
- •
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking any other drugs
When using this product
-
-
- •
- avoid alcoholic beverages
- •
- take only at bedtime
Stop use and ask a doctor if
-
-
- •
- sleeplessness persists continuously for more than two weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
-
-
- •
- adults and children 12 years of age and over: take one tablet 30 minutes before going to bed; take once daily or as directed by a doctor
- •
- children under 12 years of age: do not use
Other information
-
-
- •
- store at 68°-77°F (20°-25°C)
- •
- retain in carton until time of use
- •
- see carton end panel for lot number and expiration date
Inactive ingredients
anhydrous dibasic calcium phosphate, dibasic calcium phosphate dihydrate, FD&C blue no. 1 aluminum lake, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
Questions or comments?
1-888-423-0139
Principal Display Panel
TopCare® health
COMPARE TO UNISOM® SLEEPTABS® ACTIVE INGREDIENT
NIGHTTIME
Sleep Aid
DOXYLAMINE SUCCINATE TABLETS, 25 mg
NIGHTTIME SLEEP-AID
• Clinically Tested, Proven Effective
• One Tablet Per Dose
32 TABLETS
actual size