Indications:
Relieves:
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
Warnings
Directions:
- Adults 60years of age and over - 1-2 tablets every 4 hours. Not more than 12 tablets in 24 hours
- Adults under 60 years- 1-4 tablets every4 hours. Not more than 24 tablets in 24 hours
- Dissolve tabelt completely in water before drinking.
- DO NOT EXCEED RECOMMENDED DOSE. Not recommended for children.
Other Information:
- each tablet contains: sodium 178 mg
- store at room temperature 15 °- 30 °C (59 °- 86 °F).
In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
Questions or Comments
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
call 804-270-4498, 8.30 am-4.30 pm ET, Monday - Friday
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
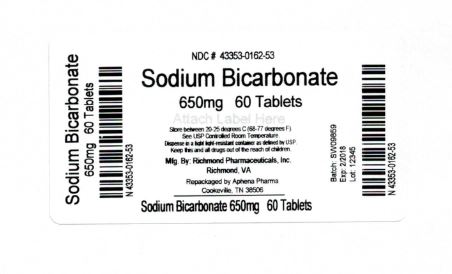
| Count | 650 mg |
| 60 | 43353-162-53 |
| 90 | 43353-162-60 |
| 120 | 43353-162-70 |
| 180 | 43353-162-80 |
| 200 | 43353-162-85 |
| 360 | 43353-162-94 |
Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20171213JH