SIL-K PAD Description
SIL-K PAD is intended for non-invasive management of old and new hypertrophic or keloid scars resulting from burns, surgical procedures or trauma wounds.
Each SIL-K PAD contains: Silicone, Polydimethyl hydrogenmethyl siloxane, Polydimethylsiloxane, and Polydimethylsiloxane hydrogen terminated.
SIL-K PAD - Clinical Pharmacology
The exact mechanism of silicone gel in improving the appearance of scar tissue remains unknown. However, numerous mechanisms have been suggested to explain the efficacy of silicone gel, including hydration, pressure, temperature, oxygen transmission and silicone absorption. There is some evidence that the treatment affects the stratum corneum and, by reducing evaporation, restores better homeostasis in the tissue. In keloid and hypertrophic scarring, the stratum corneum allows more evaporation of water from the underlying tissue than occurs in normal skin. Silicone pads may prevent this, keeping the stratum corneum in optimal hydration and protecting the skin from environmental hazards, both of which can reduce abnormal scarring. The gel may also affect the stratum corneum by inhibiting mast cell activity, diminishing edema, vasodilatation and excessive extracellular matrix formation but the simple changes in temperature, pressure, oxygen tension and hydration produced by wound coverage probably constitute the main mechanism of action. Another hypothesis is that the effect of static electricity on silicone may influence the alignment of collagen deposition.
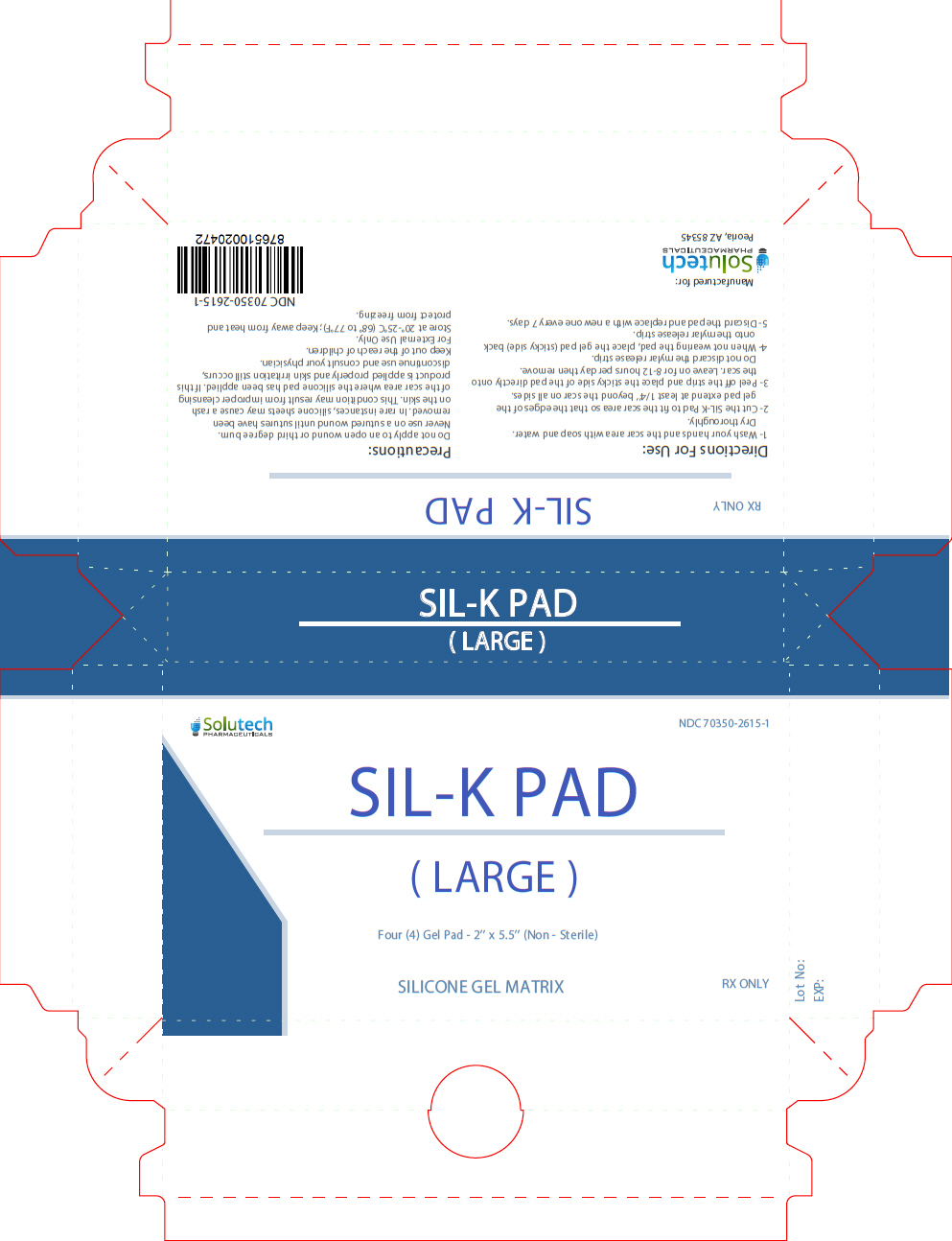
INDICATIONS AND USES
For non-invasive management of old and new hypertrophic or keloid scars resulting from burns, general surgical procedures, injuries or trauma wounds.
Contraindications
SIL-K PAD is contraindicated in patients with known hypersensitivity to silicone or any of the listed ingredients.
Warnings
For external use only. Avoid contact with eyes, lips or mucous membranes. Do not apply on areas of broken skin. Do not apply on third degree burns and open wounds. Never use on sutured wound until sutures have been removed.
Precautions
Stop use and ask a doctor if irritation develops. In rare instances, silicone sheets may cause a rash on the skin. This condition may result from improper cleansing of the scar area where the silicone pad has been applied. If this product is applied properly and skin irritation still occurs, discontinue use and consult your physician. If ingested, get medical help or contact Poison Control Center right away. KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
This medication should be used as directed by your physician during pregnancy or while breast-feeding. Consult your doctor about the risks and benefits.
Adverse Reactions
Rarely the Pads may cause transient redness stinging, burning or irritation and normally disappear on discontinuing the medication.
SIL-K PAD - Dosage and Administration
- 1-
- Wash your hands and the scar area with soap and water. Dry thoroughly.
- 2-
- Cut the SIL-K Pad to fit the scar area so that the edges of the gel pad extend at least 1/4'' beyond the scar on all sides.
- 3-
- Peel of the strip and place the sticky side of the pad directly onto the scar. Leave on for 8-12 hours per day then remove. Do not discard the mylar release strip.
- 4-
- When not wearing the pad, place the gel pad (sticky side) back onto the mylar release strip.
- 5-
- Discard the pad and replace with a new one every 7 days.