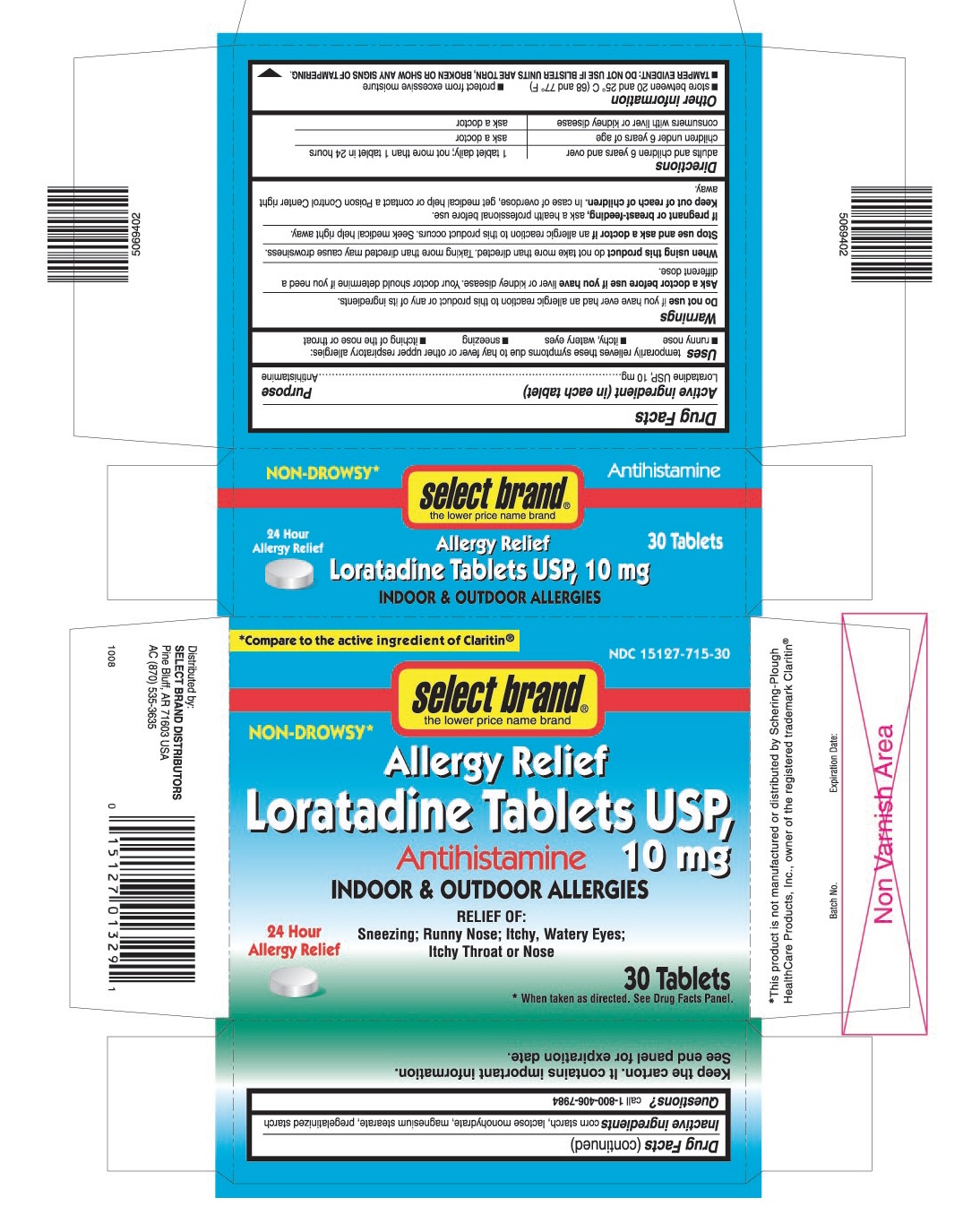
ACTIVE INGREDIENT (IN EACH TABLET)
Loratadine USP, 10 mg
USES
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
WARNINGS
Do not use
If you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
Liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
Do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
An allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding
Ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
| adults and children 6 years and over | 1 tablet daily; not more than 1 tablet in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
OTHER INFORMATION
- store between 20 and 25° C (68 and 77° F)
- protect from excessive moisture
-
TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
INACTIVE INGREDIENTS
Corn starch, lactose monohydrate, magnesium stearate, pregelatinized starch
QUESTIONS?
Call 1-800-406-7984
PRINCIPAL DISPLAY PANEL
*Compare to the active ingredient of Claritin®
NDC 15127-715-30
select brand®
NON-DROWSY٭
Allergy Relief
Loratadine Tablets USP, 10 mg
Antihistamine
INDOOR & OUTDOOR ALLERGIES
RELIEF OF:
Sneezing; Runny Nose; Itchy, Watery Eyes; Itchy Throat or Nose
24 Hour Allergy Relief
30 Tablets
٭When taken as directed. See Drug Facts Panel.
Distributed by: SELECT BRAND DISTRIBUTORS
5069402/1008