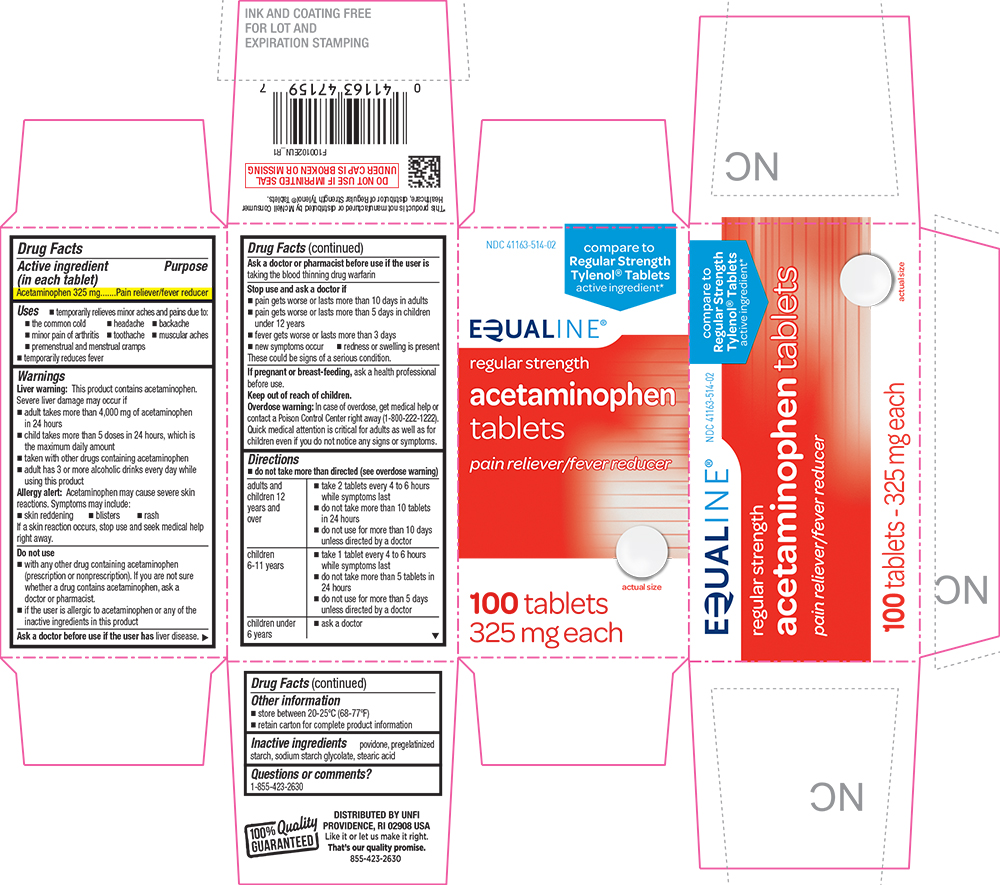
ACETAMINOPHEN REGULAR STRENGTH- acetaminophen tablet
United Natural Foods, Inc. dba UNFI
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each tablet)
Acetaminophen 325 mg
Purpose
Pain reliever/fever reducer
Uses
- temporarily relieves minor aches and pains due to:
- the common cold
- headache
- backache
- minor pain of arthritis
- toothache
- muscular aches
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if
- adult takes more than 4,000 mg of acetaminophen in 24 hours
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert
acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if the user is allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor before use if the user has liver disease.
Ask a doctor or pharmacist before use if the user is taking the blood thinning drug warfarin
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days in adults
- pain gets worse or lasts more than 5 days in children under 12 years
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Overdose warning
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
| adults and children 12 years and over |
- take 2 tablets every 4 to 6 hours while symptoms last
- do not take more than 10 tablets in 24 hours
- do not use for more than 10 days unless directed by a doctor
|
| children 6-11 years |
- take 1 tablet every 4 to 6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
- do not use for more than 5 days unless directed by a doctor
|
| children under 6 years |
|
Other information
- store between 20-25°C (68-77°F)
- retain carton for complete product information
Inactive ingredients
povidone, pregelatinized starch, sodium starch glycolate, stearic acid
Questions or comments?
1-855-423-2630
PRINCIPAL DISPLAY PANEL
Equaline®
NDC 41163-514-02
compare to Regular Strength Tylenol® Tablets active ingredient*
regular strength
acetaminophen tablets
pain reliever/fever reducer
100 tablets - 325 mg each
actual size
United Natural Foods, Inc. dba UNFI
