Uses
washes the eye to help relieve
- irritation
- stinging
- discomfort
- itching;
by removing
- loose foreign material
- air pollutants (smog or pollen)
- chlorinated water
Warnings
Directions
Remove contact lenses before using.
- Do not touch tip of container to any surface to avoid contamination.
- Replace cap after use.

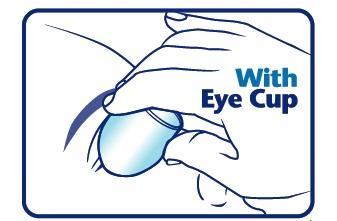
When using an eye cup
- rinse the cup with Pure-aid Eye Wash immediately before each use
- avoid contamination of the rim and inside surfaces of the cup
- fill the cup half full with Pure-aid Eye Wash Solution and apply the cup to the affected eye(s), pressing tightly to prevent spillage
- tilt the head backward. Open eyelids wide and rotate eyeball to thoroughly wash the eye
- rinse cup with clean water after each use
Other information
- store at room temperature
- keep tightly closed
- use before expiration date marked on the carton or bottle
