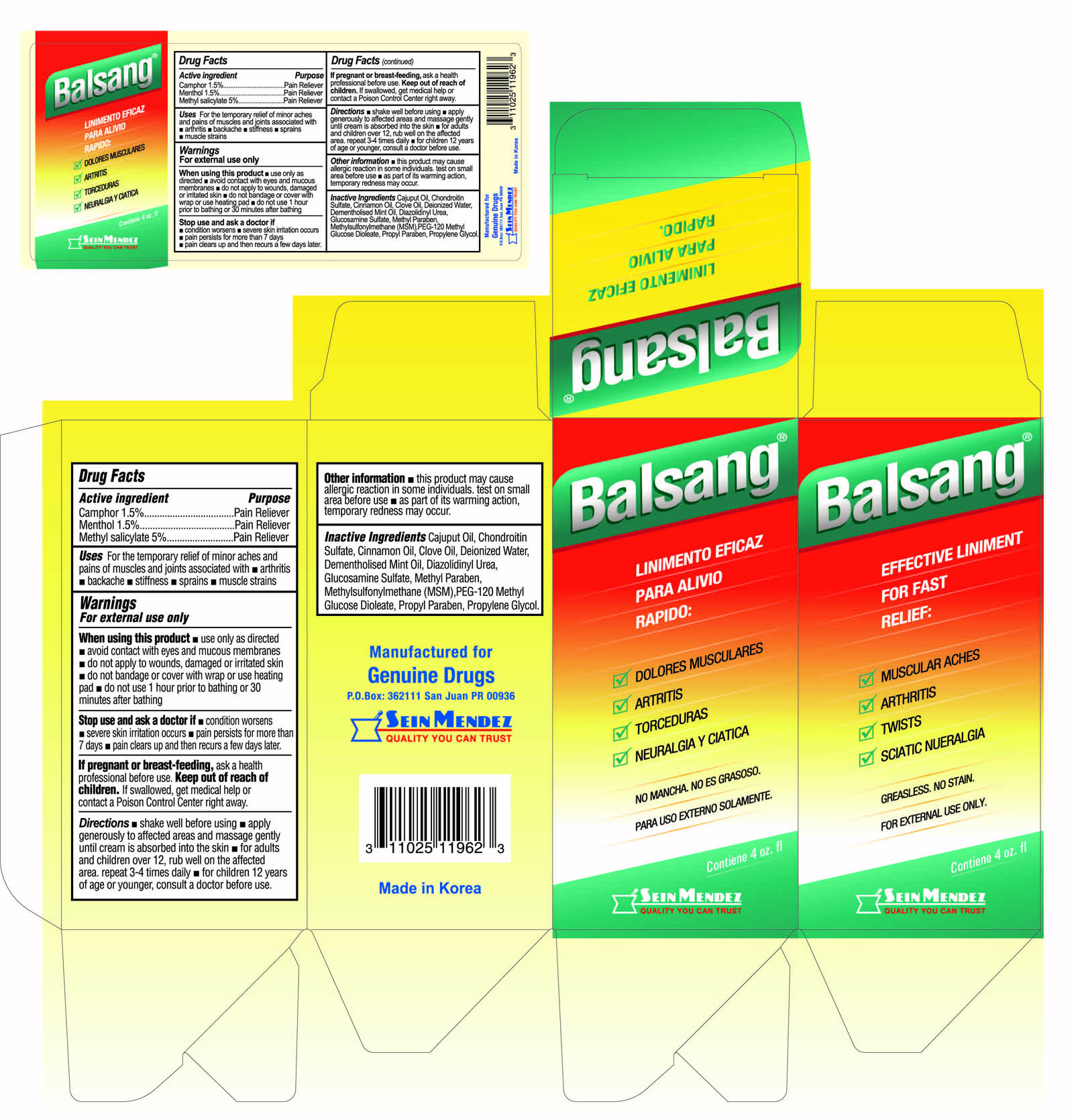
Uses
For the temporarily relieves minor aches and pains of muscle and joints associated with
- arthritis
- backache
- stiffness
- sprains
- muscle strains
For external use only.
When using this product
- use only as directed
- avoid contact with eyes and mucous membranes
- do not apply to wounds, damaged or irritated skin
- do not bandage or cover with wrap or use heating pad
- do not use 1 hour prior to bathing or 30 minutes after bathing
Stop use and ask a doctor if
- condition worsens
- severe skin irritation occurs
- pain persists for more than 7 days
- pain clears up and then reocurs a few days later.
Directions
- shake well before using
- apply generously to affected areas and massage gently until cream is absorbed into the skin
- for adults and children over 12, rub well on the affected area. repeat 3-4 times daily
- for children 12 years of age or younger, consult a doctor before use
Other information
- this product may cause allergic reactions in some individuals. test on small area before use
- as part of its warming action, temporary redness may occur.