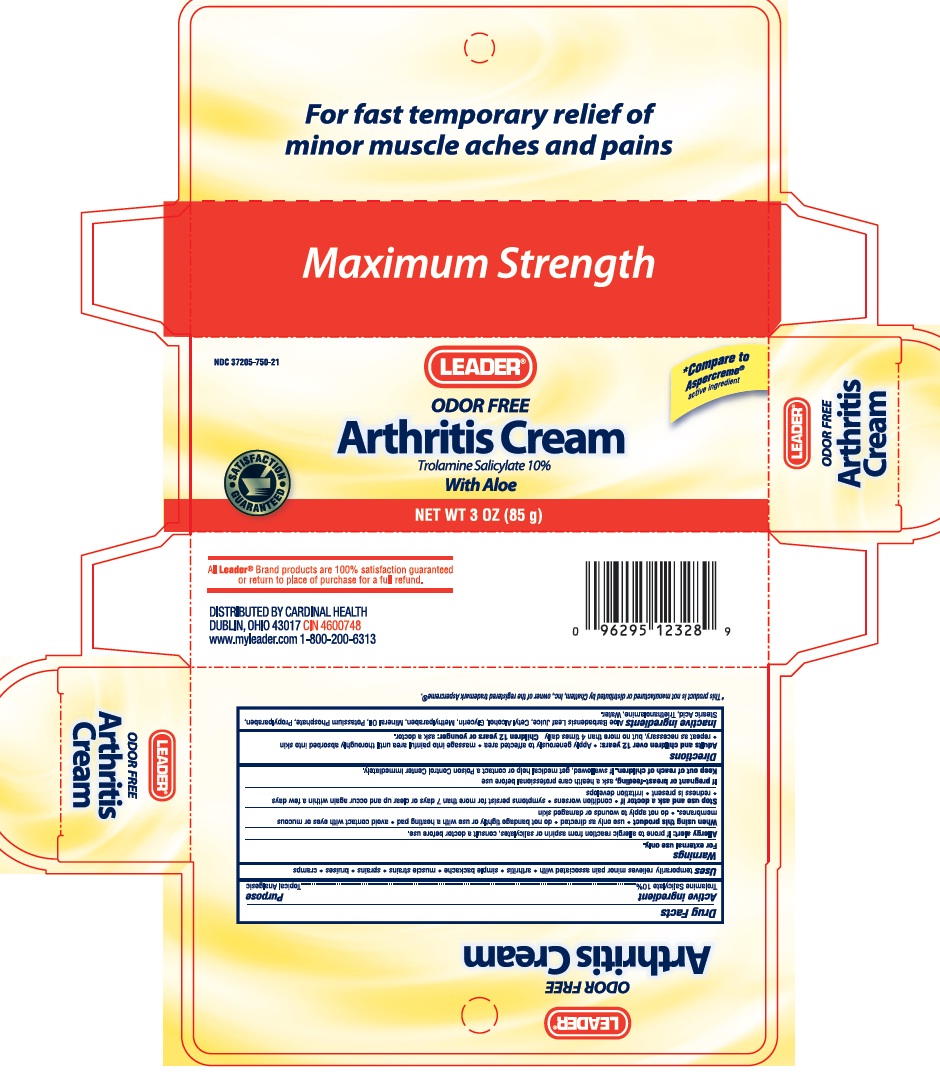
Uses
Temporarily relieves minor pain associated with: arthritis, simple backache, muscle strains, sprains, bruises, and cramps.
Warnings
For external use only.
Allergy alert: if prone to allergic reaction from aspirin or salicylate, contact a doctor before use
When using this product: use only as directed, do not bandage tightly or use with a heating pad, avoid contact with eyes or mucous membranes, and do no apply to wounds or damaged skin
Stop use and ask doctor if: condition worsens, symptoms persist for more than 7 days or clear up and occur again within a few days, redness present, or irritation develops
If pregnant or breast-feeding, ask a health care professional before use
Directions
Adults and children over 12 years of age: apply generously to affected area, massage into painful area until thoroughly absorbed into skin, repeat as necessary, but not more than 4 times daily. Children 12 years or younger: ask a doctor.
Inactive ingredients
Aloe Barbadensis Leaf Juice, Cetyl Alcohol, Glycerin, Methylparaben, Mineral Oil, Potassium Phosphate, Propylparaben, Stearic Acid, Triethanolamine, Water.