ZYLASTXP ANTISEPTIC- benzethonium chloride lotion
Innovative BioDefense
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
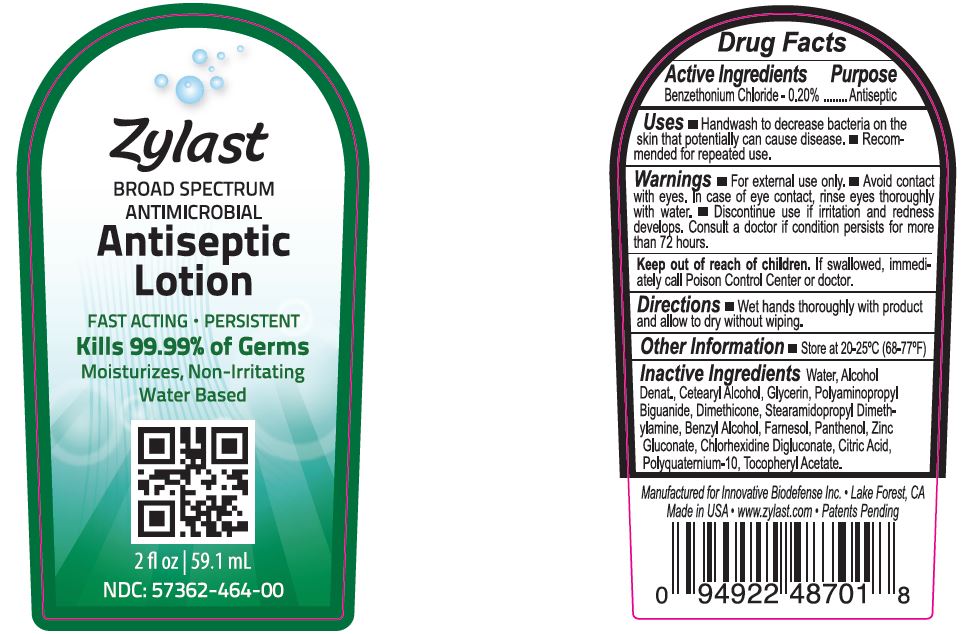
Benzethonium Chloride - 0.2%
Discontinue use if irritation and redness develops. Consult a doctor if condition persists for more than 72 hours.
If swallowed, immediately call Poison Control Center of doctor.
Wet hands thoroughly with product and allow to dry without wiping.
For external use only. Avoid contact with eyes. In case of eye contact, rinse eyes thoroughly with water. Discontinue use if irriation and redness develops. Consult a doctor if condition persists for more than 72 hours.
Water, Alcohol Denat., Cetearly Alcohol, Glycerin, Polyaminopropyl Biguanide, Dimethicone, Stearamidopropyl Dimethylamine, Benzyl Alcohol, Farnesol, Pantenol, Zinc Gluconate, Chlohexidine Digluconate, Citric Acid, Polyquaternium-10, Tocopheryl Acetate.
Handwash to decrease bacteria on the skin that potentially can cause disease. Recommended for repeated use.
NDC 57362-464-00
Zylast Antiseptic Lotion 2oz
Broad Spectrum
Antimicrobial