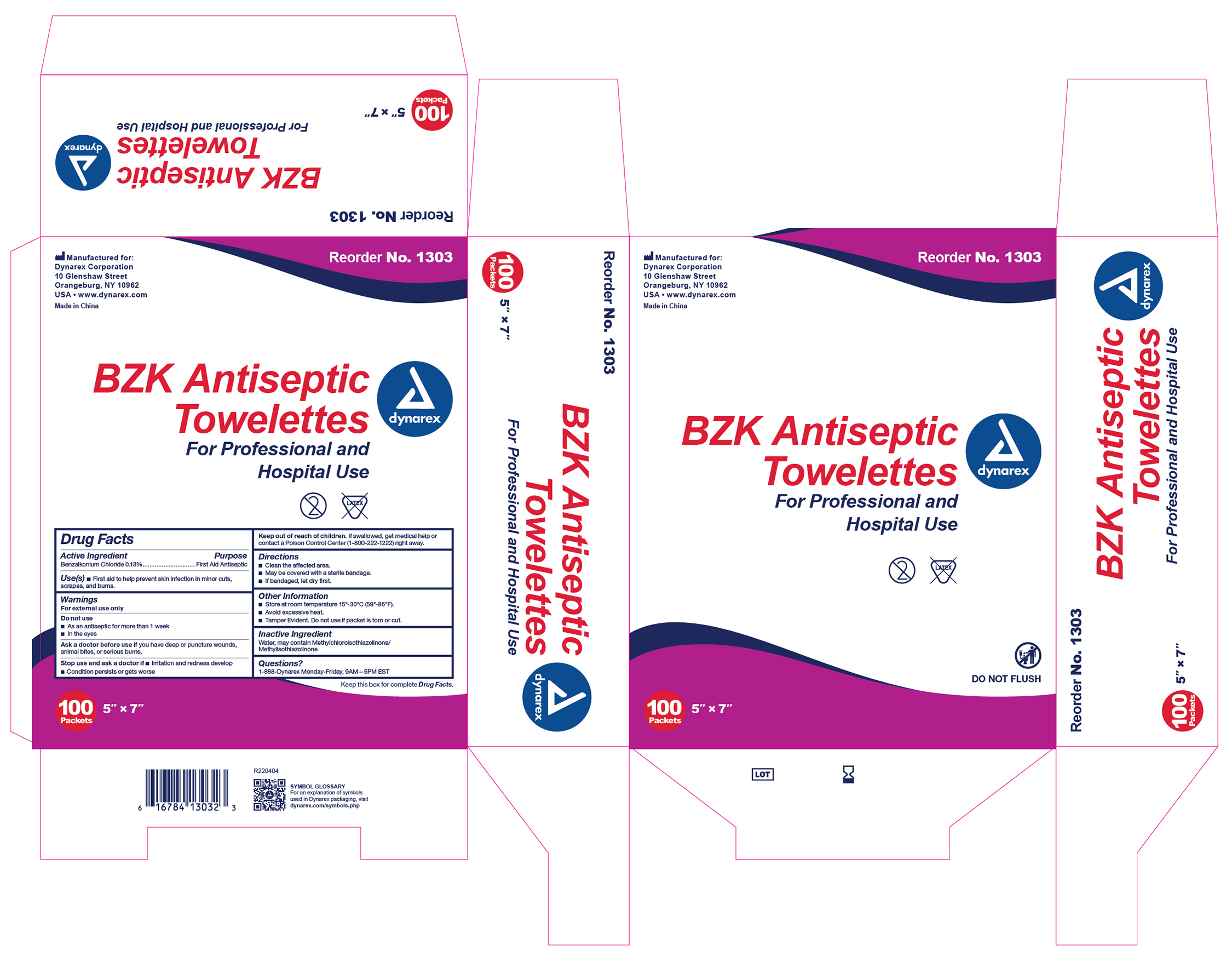
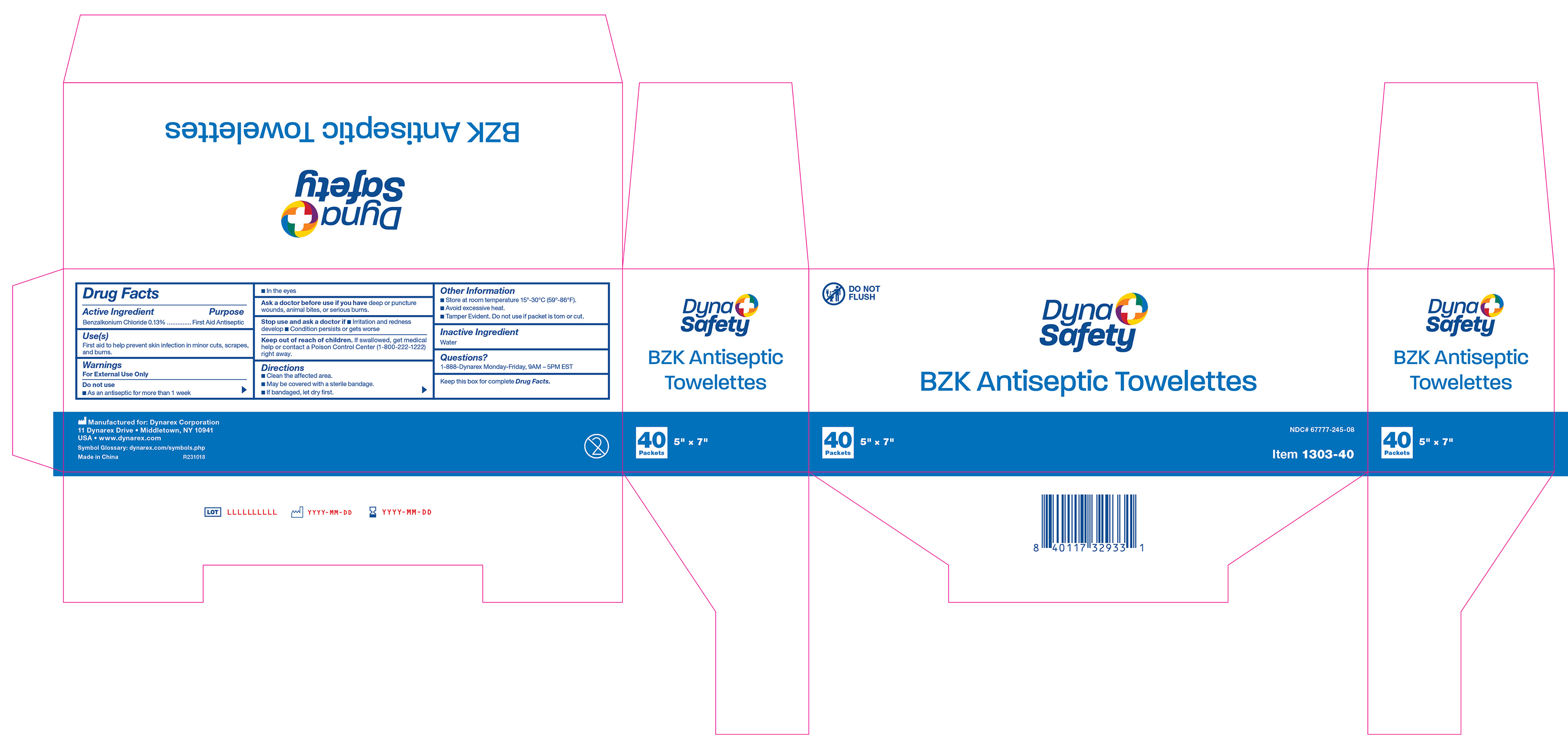
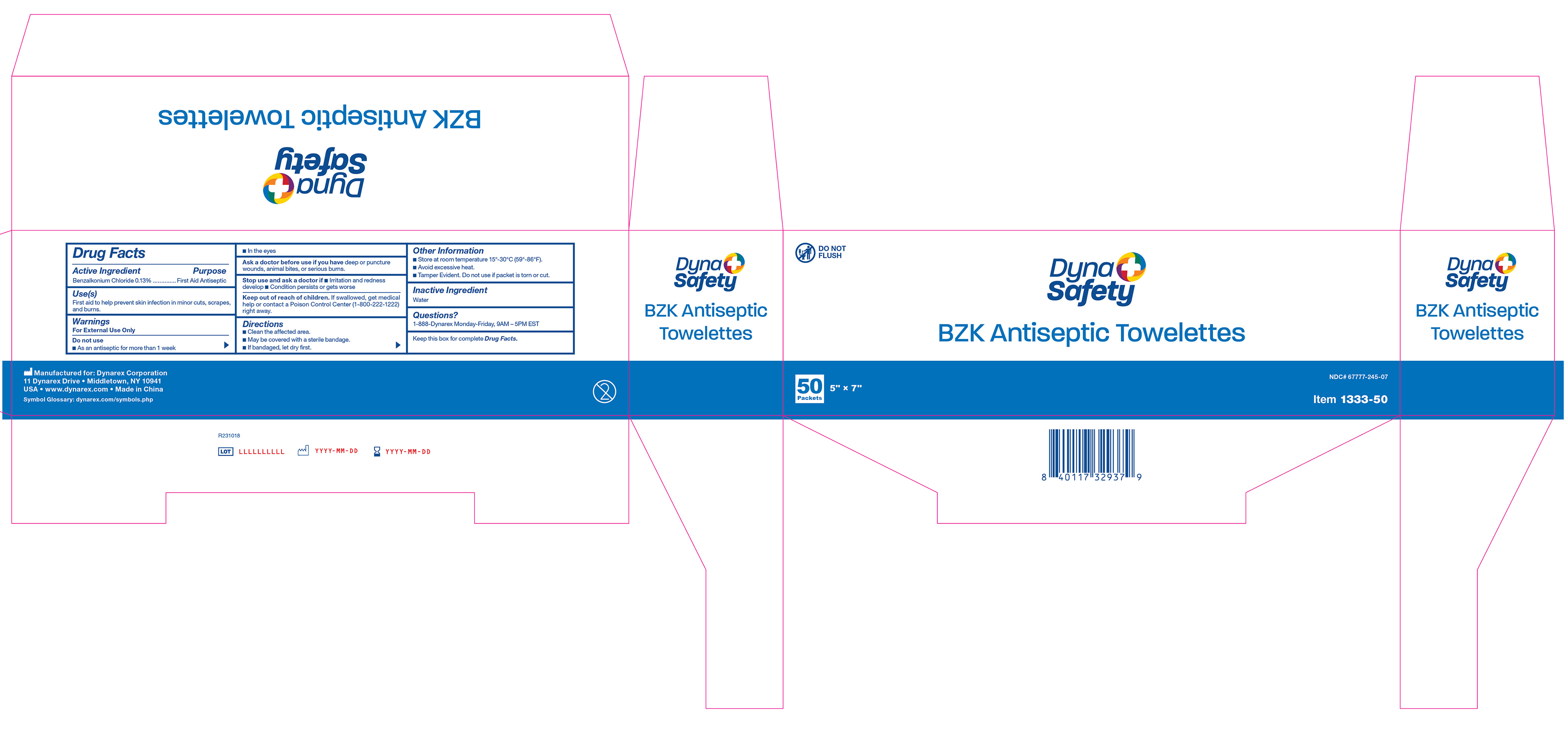
Active Ingredient
Benzalkonium Chloride 0.13%
Purpose
First Aid Antiseptic
Use(s)
First aid to help prevent skin infection in minor cuts, scrapes, and burns.
Warnings
For External Use Only
Do not use:
• As an antiseptic for more than 1 week
• In the eyes
Ask a doctor before use if you have
Deep or puncture wounds, animal bites, or serious burns.
Stop use and ask a doctor if
• Irritation and redness develop
• Condition persists or gets worse
Keep Out Of Reach Of Children.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
• Clean the affected area.
• May be covered with a sterile bandage.
• If bandaged, let dry first.
Other Information
• Store at room temperature 15º-30ºC (59º-86ºF).
• Avoid excessive heat.
• Tamper Evident. Do not use if packet is torn or cut.
Inactive ingredients
Water
Questions?
1-888-DYNAREX Monday - Friday, 9AM - 5PM EST
Label
 1303 Label
1303 Label
Label
Label
 1331
1331
Label
 1332
1332
Label
 1333
1333
Label

Label 1303UB-10
 1303UB-10
1303UB-10
Label 1303-40
 1303-40
1303-40
Label 1333-50
 1333-50
1333-50