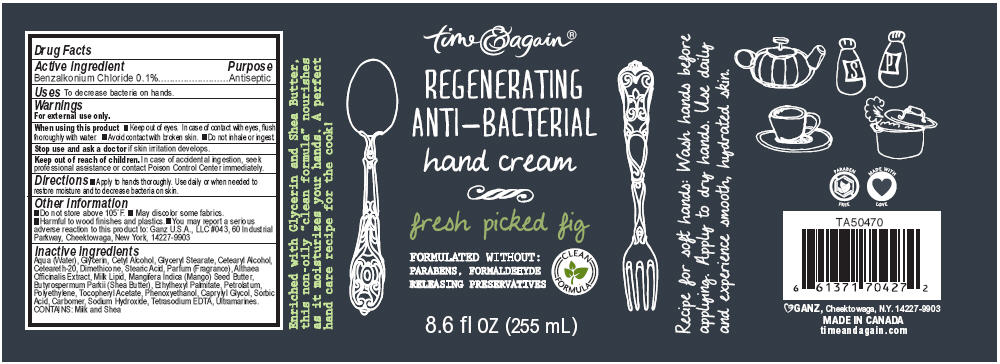
GOURMET KITCHEN REGENERATING ANTI-BACTERIAL FRESH PICKED FIG- benzalkonium chloride lotion
GOURMET KITCHEN REGENERATING ANTI-BACTERIAL SWEET PLUM- benzalkonium chloride lotion
GANZ U.S.A., LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Benzalkonium Chloride 0.1%
Uses
To decrease bacteria on hands.
Warnings
For external use only.
When using this product
- Keep out of eyes. In case of contact with eyes, flush thoroughly with water.
- Avoid contact with broken skin.
- Do not inhale or ingest.
Stop use and ask a doctor if skin irritation develops.
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact Poison Control Center immediately.
Directions
- Apply to hands thoroughly. Use daily or when needed to restore moisture and to decrease bacteria on skin.
Other information
- Do not store above 105°F.
- May discolor some fabrics.
- Harmful to wood finishes and plastics.
- You may report a serious adverse reaction to this product to: Ganz U.S.A., LLC #043, 60 Industrial Parkway, Cheektowaga, New York, 14227-9903
Inactive ingredients
Aqua (Water), Glycerin, Cetyl Alcohol, Glyceryl Stearate, Cetearyl Alcohol, Ceteareth-20, Dimethicone, Stearic Acid, Parfum (Fragrance), Althaea Officinalis Extract, Milk Lipid, Mangifera Indica (Mango) Seed Butter, Butyrospermum Parkii (Shea Butter), Ethylhexyl Palmitate, Petrolatum, Polyethylene, Tocopheryl Acetate, Phenoxyethanol, Caprylyl Glycol, Sorbic Acid, Carbomer, Sodium Hydroxide, Tetrasodium EDTA, Ultramarines. CONTAINS: Milk and Shea
PRINCIPAL DISPLAY PANEL - 255 mL Bottle Label
time & again®
REGENERATING
ANTI-BACTERIAL
hand cream
fresh picked fig
FORMULATED WITHOUT:
PARABENS, FORMALDEHYDE
RELEASING PRESERVATIVES
CLEAN
FORMULA
8.6 fl oz (255 mL)
PRINCIPAL DISPLAY PANEL - 255 mL Bottle Label
time & again®
REGENERATING
ANTI-BACTERIAL
hand cream
sweet wild plum
FORMULATED WITHOUT:
PARABENS, FORMALDEHYDE
RELEASING PRESERVATIVES
CLEAN
FORMULA
8.6 fl oz (255 mL)