Ask a doctor before use if you have
- vaginal itching and discomfort for the first time
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- been exposed to human immunodeficiency virus (HIV) that causes AIDS
When using this product
- do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted disease (STDs).
- do not have vaginal intercourse
- mild increase in vaginal burning, itching or irritation may occur
Stop use and ask a doctor if
- symptoms do not get better after 3 days
- symptoms last more than 7 days
- you get a rash or hives, abdominal pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- before using this product read the enclosed brochure and instructions on foil packet for complete directions and information
- adults and children 12 years and over: 1.Open the foil packet just before use and remove purple cap. 2.Insert entire contents of applicator into the vagina at bedtime. Throw applicator away after use.
- children under 12 years of age: ask a doctor
Other information
- this product is a 1-dose treatment, most women do not experience complete relief of their symptoms in just one day. Most women experience some relief within one day and complete relief of symptoms within 7 days.
- if you have questions about vaginal yeast infections, consult your doctor
- store at 20 - 25°C (68 - 77°F)
- see end flap of carton for lot number and expiration date
Do not use if sealed foil packet torn, open, or incompletely sealed.
1-DOSE TREATMENT
TIOCONAZOLE OINTMENT 6.5%
VAGINAL ANTIFUNGAL
DIRECTIONS FOR USE
REMOVE PURPLE CAP from top of applicator with a pull-twist action.
Insert the applicator
PLEASE READ EDUCATIONAL BROCHURE FOR ADDITIONAL INFORMATION.
4260000 P6
DISTRIBUTED BY
PERRIGO
ALLEGAN, MI 48010 U.S.A.
1-DOSE TREATMENT
TIOCONAZOLE OINTMENT 6.5%
VAGINAL ANTIFUNGAL
DIRECTIONS FOR USE
- Tear open foil packet just before using. It is best to use at bedtime.
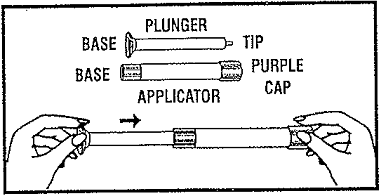
- Remove applicator and plunger from packet. Applicator is prefilled with vaginal ointment.
- While firmly holding the purple-capped end of the applicator, push the tip of the plunger into the base of applicator.

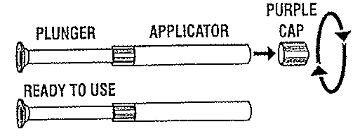
REMOVE PURPLE CAP from top of applicator with a pull-twist action.

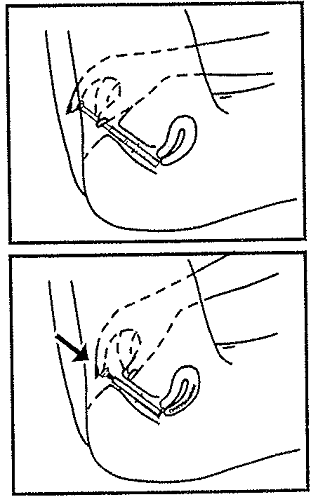
Insert the applicator
- Lie on your back with your knees bent. Gently insert applicator into the vagina as far as it will go comfortably.
- Push the plunger into the applicator until it will go no farther. Withdraw the applicator and plunger and dispose of it in the wastebasket. Do not flush.
- DO NOT USE TAMPONS while using this medicine. Use sanitary napkins instead.

PLEASE READ EDUCATIONAL BROCHURE FOR ADDITIONAL INFORMATION.
4260000 P6
DISTRIBUTED BY
PERRIGO
ALLEGAN, MI 48010 U.S.A.