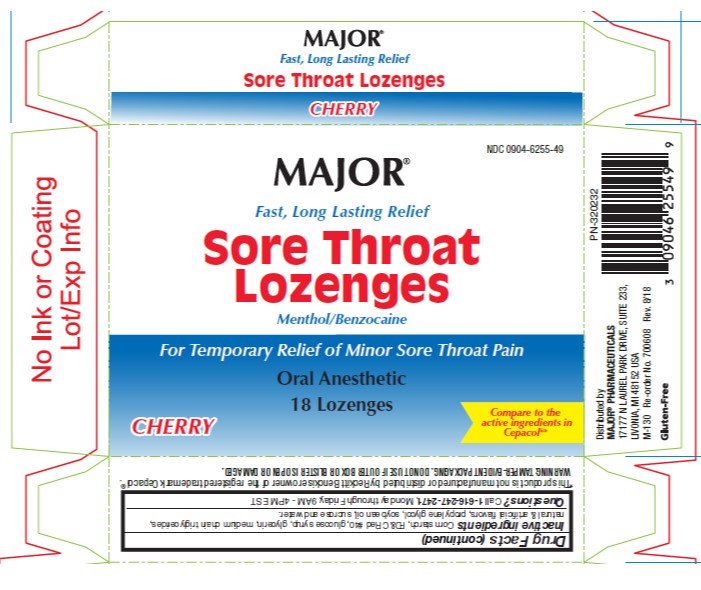
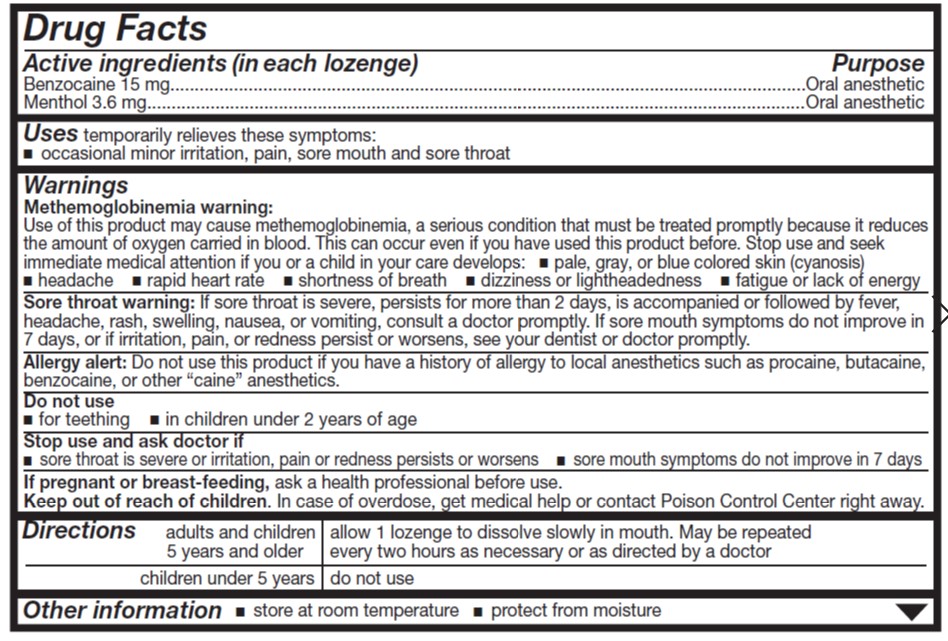
Uses
temporary relief of occasional
- sore throat
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Warnings
Allergy alert
- Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other 'caine' anesthetics.
Sore throat warning
- If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting consult a doctor promptly.
Stop use and ask a doctor or dentist if
- sore mouth symptoms do not improve in 7 days
- irritation, pain or redness persists or worsens
- swelling, rash or fever develops
Directions
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: ask a doctor
Other information
- tamper evident packaging: Do not use if outer box or blister is open or damaged.
- store at room temperature
- protect contents from moisture