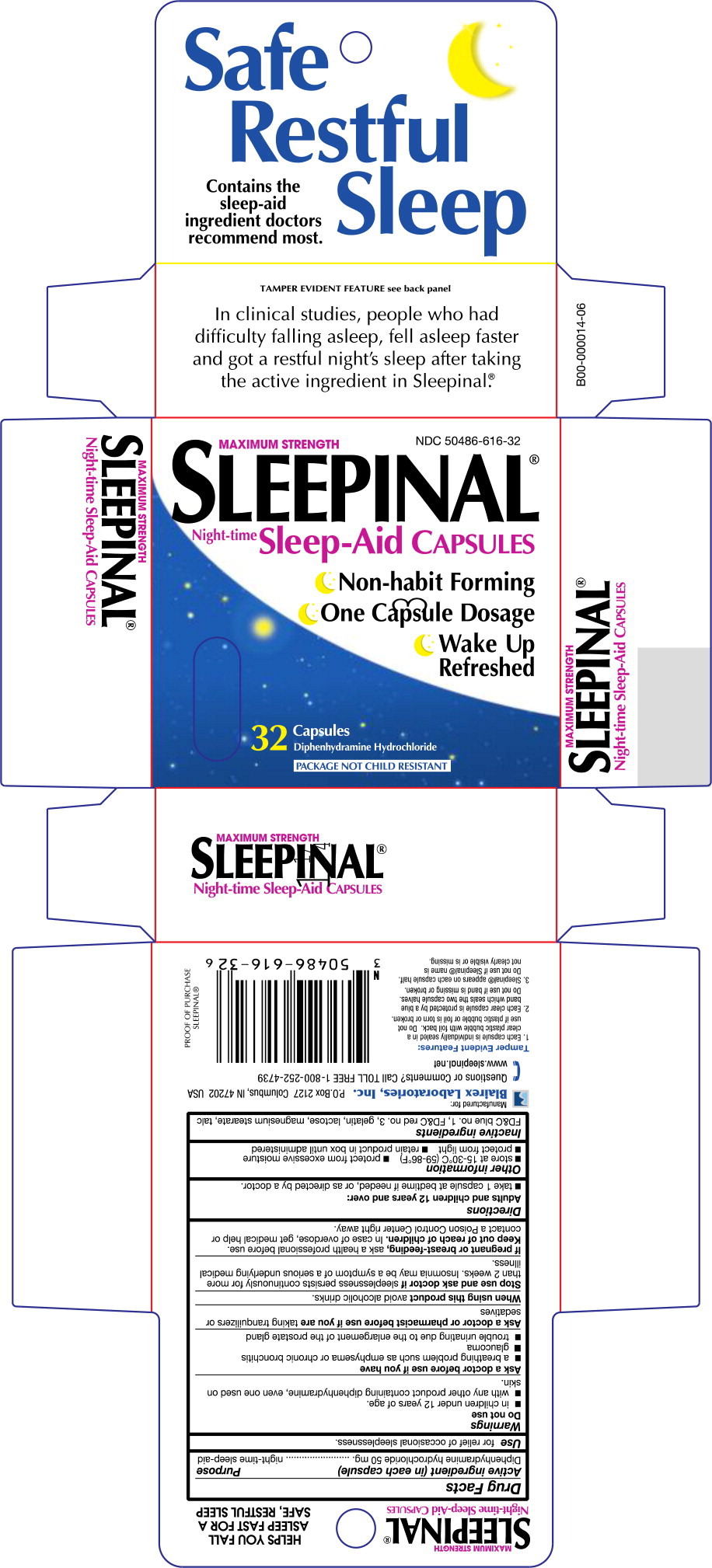
SLEEPINAL- diphenhydramine hydrochloride capsule
Blairex Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each capsule)
Diphenhydramine hydrochloride 50 mg
Purpose
night-time sleep-aid
Use
for relief of occasional sleeplessness.
Warnings
Do not use
- in children under 12 years of age.
- with any other product containing diphenhydramine, even one used on skin.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to the enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives
When using this product avoid alcoholic drinks.
Stop use and ask doctor if sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years and over:
- take 1 capsule at bedtime if needed, or as directed by a doctor.
Other information
- store at 15-30°C (59-86°F)
- protect from excessive moisture
- protect from light
- retain product in box until administered
Inactive ingredients
FD&C blue no. 1, FD&C red no. 3, gelatin, lactose, magnesium stearate, talc
Manufactured for:
Blairex Laboratories, Inc.
P.O. Box 2127 Columbus, IN 47202 USA
 Questions or Comments?
Call TOLL FREE 1-800-252-4739
Questions or Comments?
Call TOLL FREE 1-800-252-4739
www.sleepinal.net
MAXIMUM STRENGTH
Sleepinal
®
Night-time Sleep-Aid CAPSULES
NDC 50486-616-32
Non-habit Forming
One Capsule Dosage
Wake Up Refreshed
32 Capsules
Diphenhydramine Hydrochloride
PACKAGE NOT CHILD RESISTANT
MAXIMUM STRENGTH
Sleepinal
®
Night-time Sleep-Aid CAPSULES
NDC 50486-616-16
Non-habit Forming
One Capsule Dosage
Wake Up Refreshed
16 Capsules
Diphenhydramine Hydrochloride
Blairex Laboratories, Inc.
 Questions or Comments?
Questions or Comments?