PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
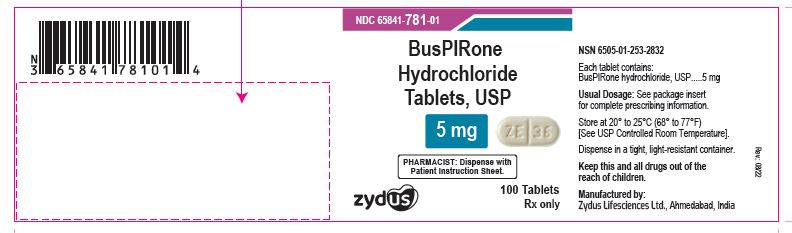
NDC 65841-781-01 in bottle of 100 tablets
Buspirone Hydrochloride Tablets USP, 5 mg
Rx only
100 tablets
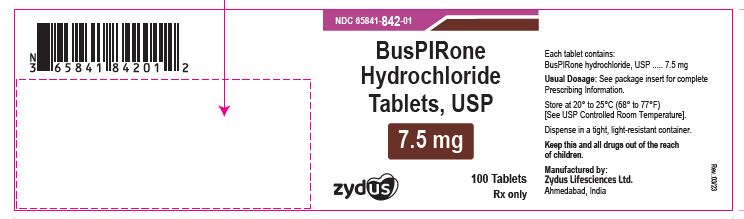
NDC 65841-842-01 in bottle of 100 tablets
Buspirone Hydrochloride Tablets USP, 7.5 mg
Rx only
100 tablets
NDC 65841-782-01 in bottle of 100 tablets
Buspirone Hydrochloride Tablets USP, 10 mg
Rx only
100 tablets

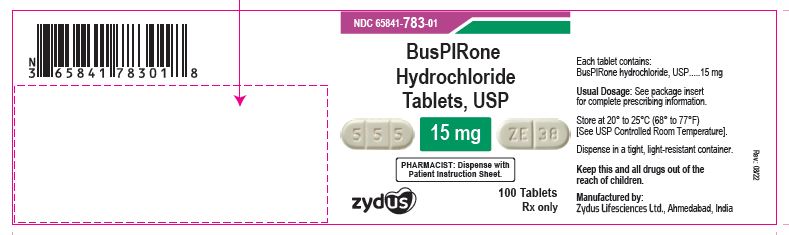
NDC 65841-783-01 in bottle of 100 tablets
Buspirone Hydrochloride Tablets USP, 15 mg
Rx only
100 tablets
NDC 65841-784-14 in bottle of 60 tablets
Buspirone Hydrochloride Tablets USP, 30 mg
Rx only
60 tablets
