Uses
- •
- For the temporary relief of burning, irritation and discomfort due to dryness of the eye or exposure to wind or sun.
- •
- May be used as a protectant against further irritation or to relieve dryness of the eye.
WARNINGS
- •
- For external use only.
- •
- To avoid contamination, do not touch tip of vial to any surface.
- •
- Do not use if solution changes color or becomes cloudy.
- •
- Do not use if carton of unit dose vial is broken or damaged.
- •
- Do not reuse. Once vial is opened, discard.
Stop use and ask doctor if:
You experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- To open, twist and pull tab to remove.
- •
- Instill 1 or 2 drops in the affected eye(s) as needed or as directed by your eyecare professional.
INACTIVE INGREDIENT SECTION
Boric Acid, Calcium Chloride, Magnesium Chloride, Potassium Chloride, Purified Water, Sodium Borate, Sodium Chloride, Sodium Hyaluronate.
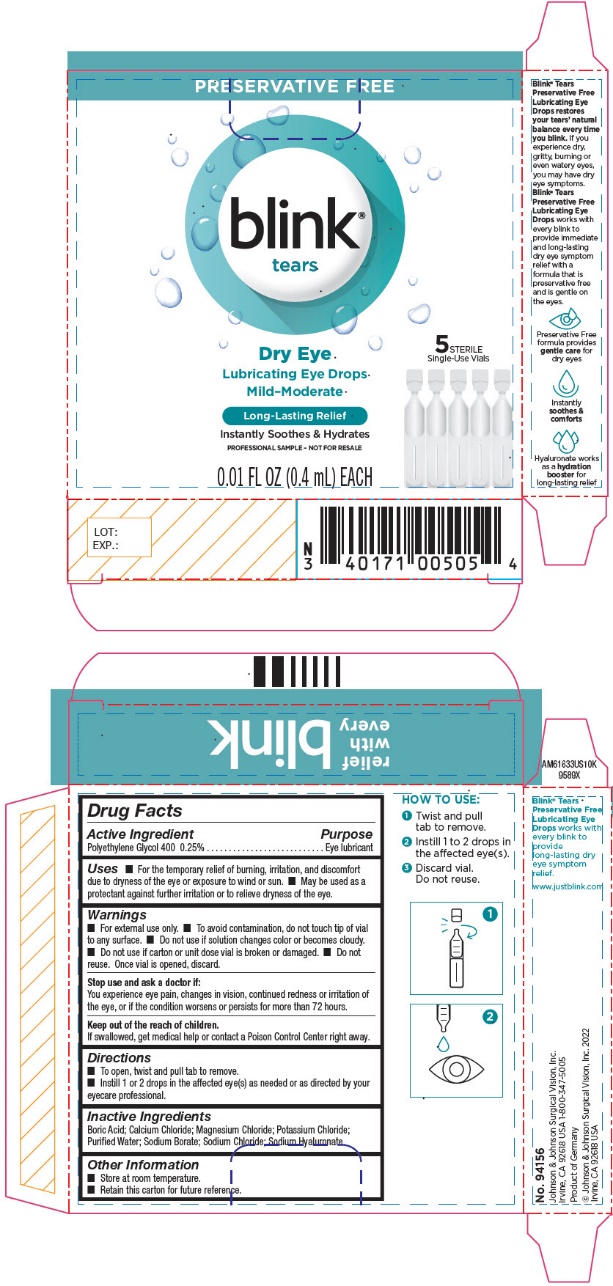
PRINCIPAL DISPLAY PANEL - 5 Vial Carton
PRESERVATIVE FREE
blink®
tears
Dry Eye
Lubricating Eye Drops
Mild-Moderate
5 STERILE
Single-Use Vials
Long-Lasting Relief
Instantly Soothes & Hydrates
PROFESSIONAL SAMPLE - NOT FOR RESALE
0.01 FL OZ (0.4 mL) EACH
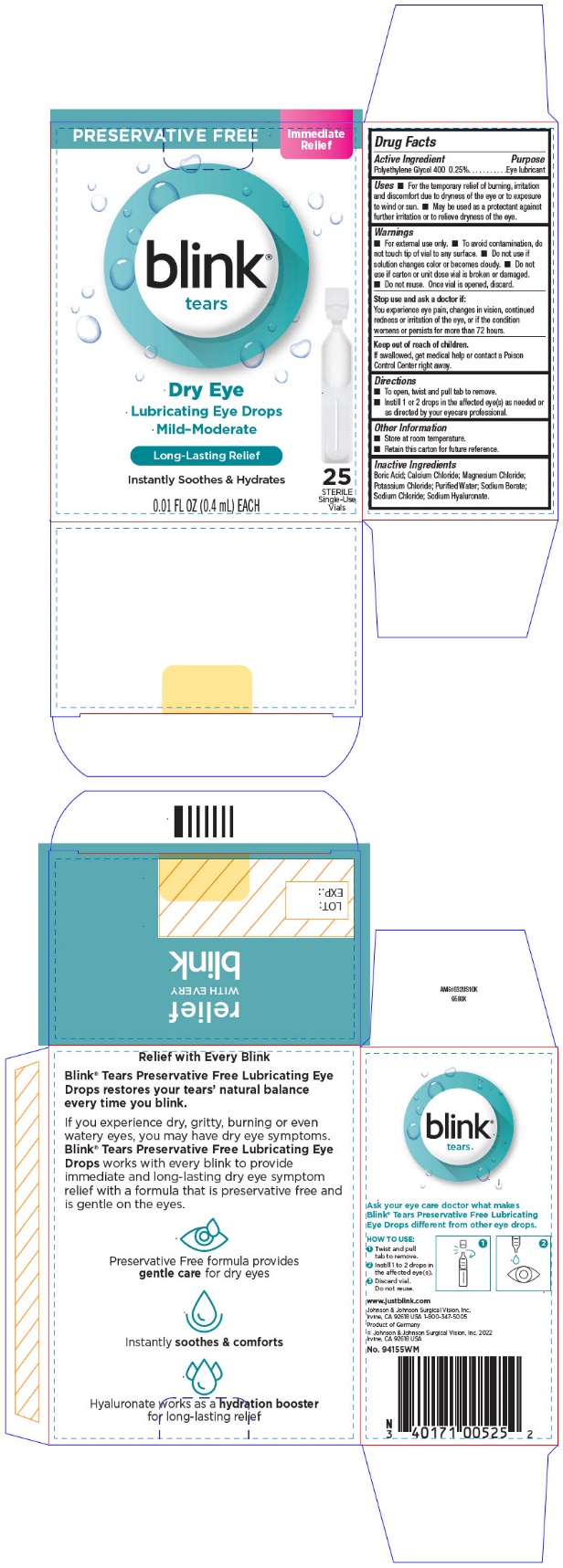
PRINCIPAL DISPLAY PANEL - 25 Vial Carton
PRESERVATIVE FREE
Immediate
Relief
blink®
tears
Dry Eye
Lubricating Eye Drops
Mild-Moderate
Long-Lasting Relief
Instantly Soothes & Hydrates
0.01 FL OZ (0.4 mL) EACH
25
STERILE
Single-Use
Vials
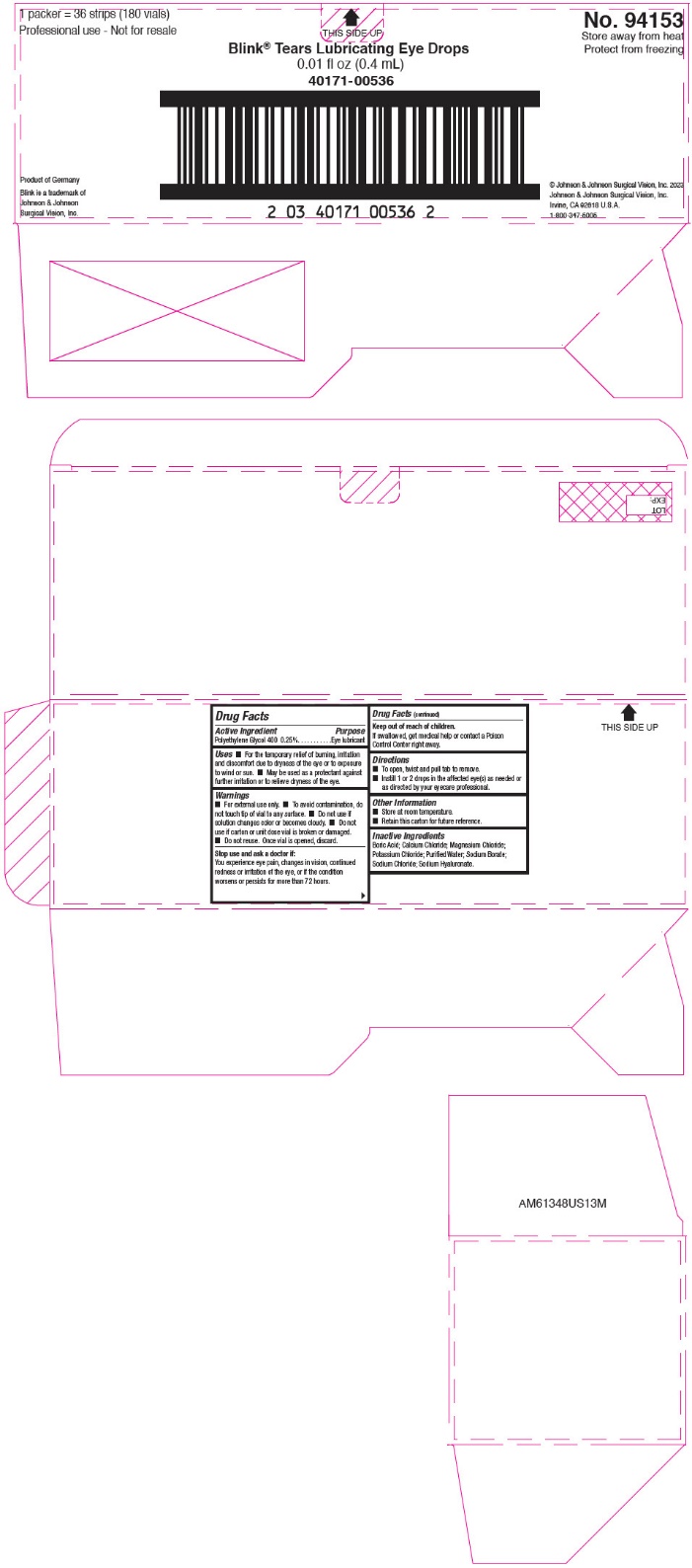
PRINCIPAL DISPLAY PANEL - 180 Vial Carton
1 packer = 36 strips (180 vials)
Professional use - Not for resale
THIS SIDE UP
No. 94153
Store away from heat
Protect from freezing
Blink® Tears Lubricating Eye Drops
0.01 fl oz (0.4 mL)
40171-00536
Product of Germany
Blink is a trademark of
Johnson & Johnson
Surgical Vision, Inc.
© Johnson & Johnson Surgical Vision, Inc. 2023
Johnson & Johnson Surgical Vision, Inc.
Irvine, CA 92618 U.S.A.
1-800-347-5005