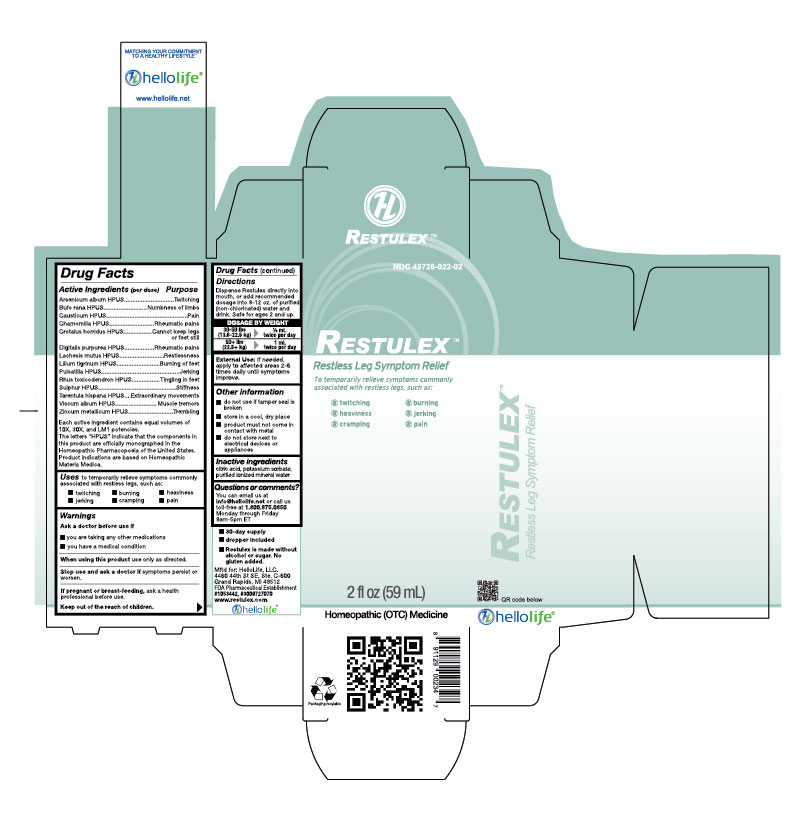
Active Ingredients (per dose)
| Arsenicum album HPUS
Bufo rana HPUS Causticum HPUS Chamomilla HPUS Crotalus horridus HPUS Digitalis purpurea HPUS Lachesis mutus HPUS Lilium tigrinum HPUS Pulsatilla HPUS Rhus toxicodendron HPUS Sulphur HPUS Tarentula hispana HPUS Viscum album HPUS Zincum metallicum HPUS Each active ingredient contains equal volumes of 10X, 30X, and LM1 potencies. The letters “HPUS” indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. Product indications are based on Homeopathic Materia Medica. |
Purpose
|
Arsenicum album HPUS..............................Twitching
Bufo rana HPUS...........................................Numbness of limbs Causticum HPUS..........................................Pain Chamomilla HPUS.......................................Rheumatic pains Crotalus horridus HPUS..............................Cannot keep legs or feet still Digitalis purpurea HPUS..............................Rheumatic pains Lachesis mutus HPUS..................................Restlessness Lilium tigrinum HPUS..................................Burning of feet Pulsatilla HPUS............................................Jerking Rhus toxicodendron HPUS..........................Tingling in feet Sulphur HPUS..............................................Stiffness Tarentula hispana HPUS..............................Extraordinary movements Viscum album HPUS....................................Muscle tremors Zincum metallicum HPUS............................Trembling |
Uses
| to temporarily relieve symptoms commonly associated with restless legs, such as:
• twitching • jerking • burning • cramping • heaviness • pain |
Warnings
Ask a doctor before use if
- you are taking any other medications
- you have a medical condition
Directions
Dispense Restulex directly into mouth, or add recommended dosage into 8-12 oz of purified (non-chlorinated) water and drink. Safe for ages 2 and up.
DOSAGE BY WEIGHT
30-50 lbs (13.6-22.6 kg) 1/2 mL twice per day
50+ lbs (22.6+ kg) 1 mL twice per day
External Use: If needed, apply to affected areas 2-6 times daily until symptoms improve.
Other information
- do not use if tamper seal is broken
- store in a cool, dry place
- product must not come in contact with metal
- do not store next to electrical devices or appliances
Questions or comments?
You can email us at
info@hellolife.net or call us toll-free at
1.800.875.0850 Monday through Friday 9am-5pm ET