Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Directions
- find right dose on chart below
- mL = milliliter
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 doses in 24 hours
| Age (yr) | Dose (mL) |
|---|---|
| children under 2 years | do not use |
| children 2 to 5 years | do not use unless directed by a doctor |
| children 6 to 11 years | 5 mL to 10 mL |
Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
Other information
- each 5 mL contains: sodium 10 mg
- store between 20-25°C (68-77°F)
- do not use if carton tape or bottle wrap imprinted with "Sealed For Your Safety" is broken or missing
- see bottom panel for lot number and expiration date
Inactive ingredients
anhydrous citric acid, carboxymethylcellulose sodium, flavors, glycerin, purified water, saccharin sodium, sodium benzoate, sodium citrate, sorbitol solution
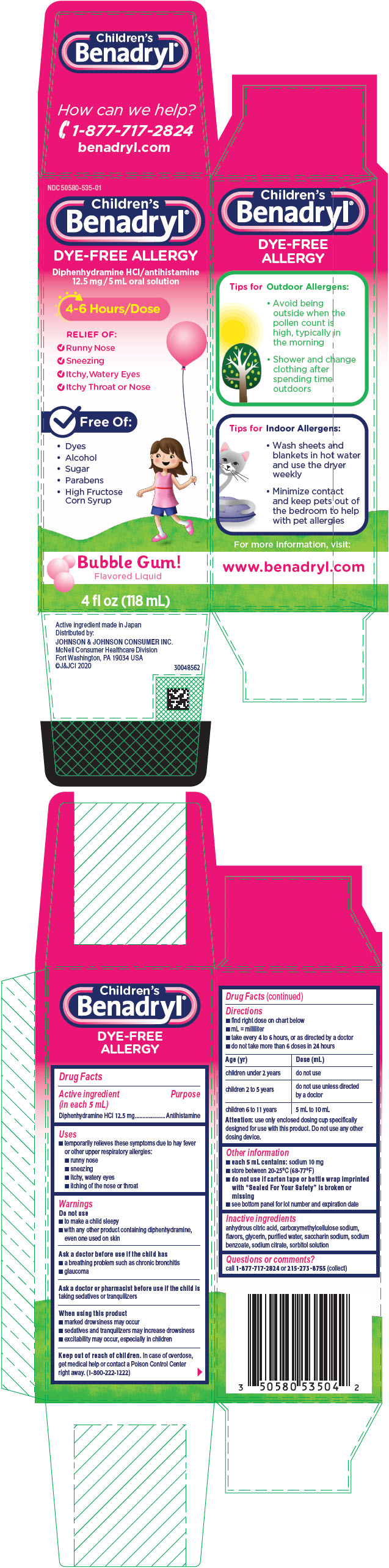
PRINCIPAL DISPLAY PANEL
NDC 50580-535-01
Children's
Benadryl
®
DYE-FREE ALLERGY
Diphenhydramine HCl/antihistamine
12.5 mg/5 mL oral solution
4-6 Hours/Dose
RELIEF OF:
- Runny Nose
- Sneezing
- Itchy,Watery Eyes
- Itchy Throat or Nose
✔ Free Of:
- Dyes
- Alcohol
- Sugar
- Parabens
- High Fructose
Corn Syrup
Bubble Gum!
Flavored Liquid
4 fl oz (118 mL)