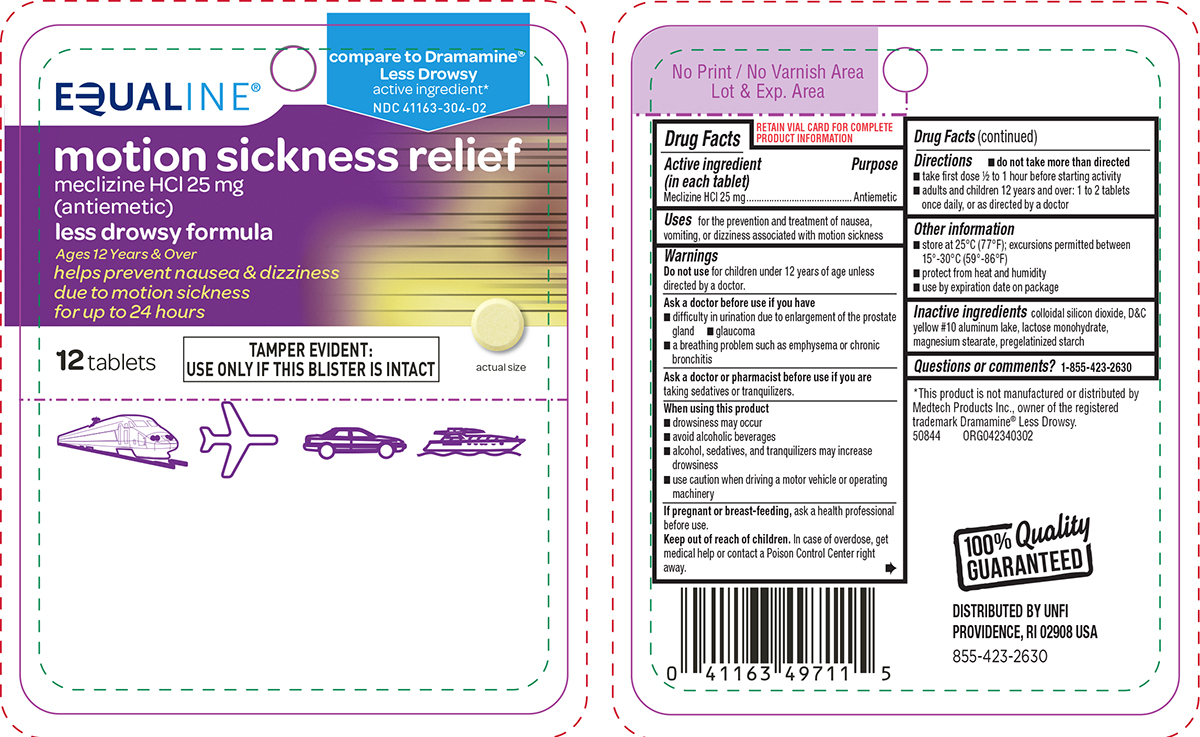
Uses
for the prevention and treatment of nausea, vomiting, or dizziness associated with motion sickness
Warnings
Ask a doctor before use if you have
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Directions
- do not take more than directed
- take first dose 1/2 to 1 hour before starting activity
- adults and children 12 years and over: 1 to 2 tablets once daily, or as directed by a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from heat and humidity
- use by expiration date on package
Inactive ingredients
colloidal silicon dioxide, D&C yellow #10 aluminum lake, lactose monohydrate, magnesium stearate, pregelatinized starch
Principal Display Panel
EQUALINE®
compare to Dramamine®
Less Drowsy
active ingredient*
NDC 41163-304-02
motion sickness relief
meclizine HCl 25 mg
(antiemetic)
less drowsy formula
Ages 12 Years & Over
helps prevent nausea & dizziness
due to motion sickness
for up to 24 hours
12 tablets
TAMPER EVIDENT:
USE ONLY IF THIS BLISTER IS INTACT
actual size
*This product is not manufactured or distributed by
Medtech Products Inc., owner of the registered
trademark Dramamine® Less Drowsy.
50844 ORG042340302
100% Quality
GUARANTEED
DISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USA
855-423-2630
Equaline 44-403