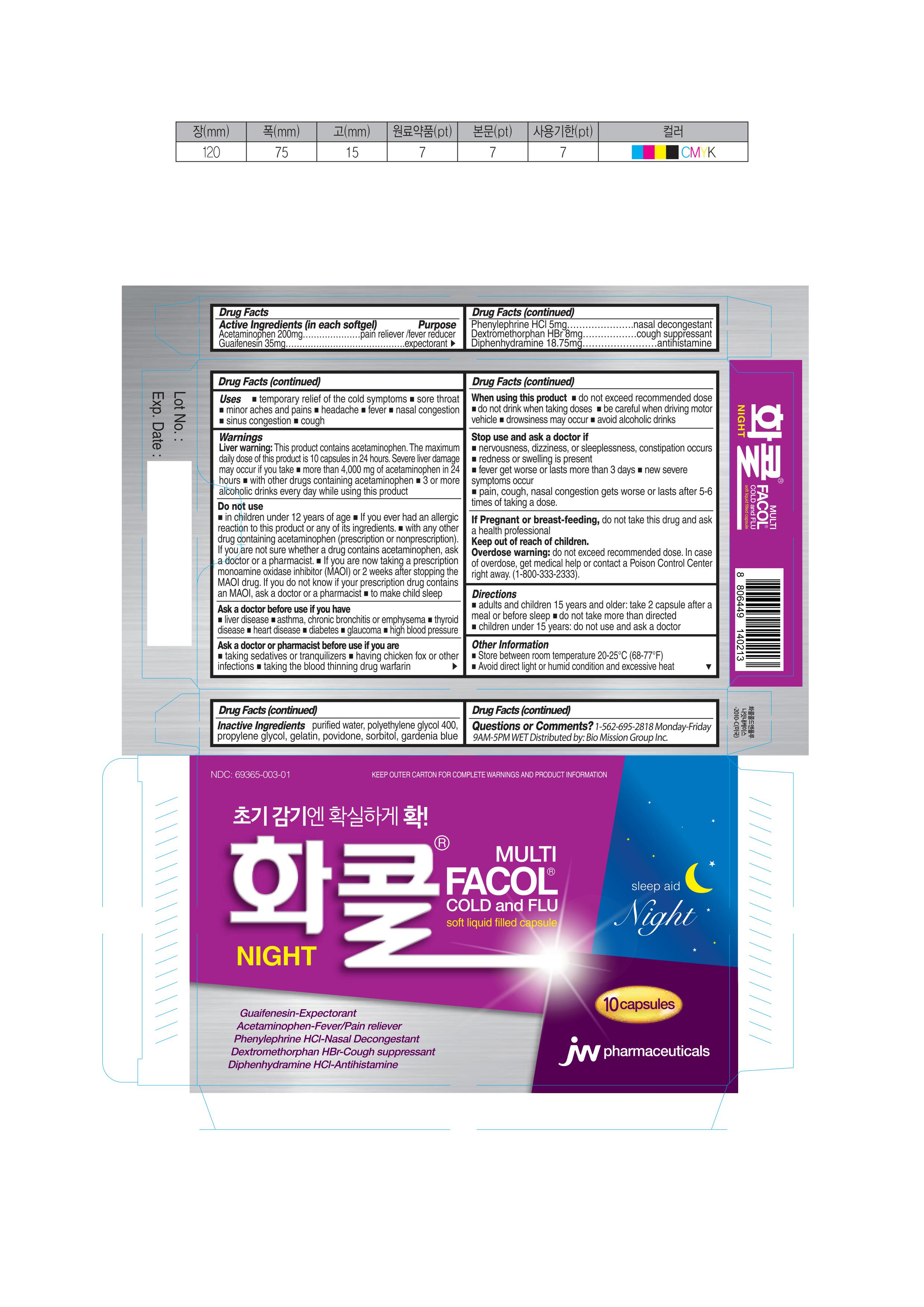
Active Ingredients (in each tablet) Purpose
Acetaminophen 200mg ………………………………………………………….………………pain reliever /fever reducer
Guaifenesin 35mg…………………………………………………………………………….……...expectorant
Phenylephrine HCl 5mg……………………….……………………………………………....….nasal decongestant
Dextromethorphan HBr 8mg…………………………………………………cough suppression
Diphenhydramine HCl 15mg………………………………………………..antihistamine
Inactive Ingredients
purified water, polyethylene glycol 400, propylene glycol, gelatin, povidone, sorbitol, gardenia blue
Uses
■ temporary relief of the following cold symptoms. ■ sore throat ■ minor aches and pains ■ headache ■ fever ■ nasal congestion ■ sinus congestion and pressure ■ cough
Warnings
Liver warning: This product contains acetaminophen. The maximum daily dose of this product is 10 caplets in 24 hours. Severe liver damage may occur if you take
■ more than 4,000 mg of acetaminophen in 24 hours
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks every day while using this product
Directions
■ adults and children 15 years and older: take 2 capsules after a meal 2 times a day ■ Do not take more than directed
■ children under 15 years: do not use and ask a doctor.
Ask a doctor before usei if
you have ■ allergic reaction ■ asthma ■ liver disease ■ pregnant or breast feeding ■ medical care ■ glaucoma ■ high blood pressure ■ thyroid disease ■ heart disease ■ diabetes
Ask a doctor or pharmacist before use if you
■ taking sedatives or tranquilizers ■ having chicken fox or other infections
■ taking the blood thinning drug warfarin
Do not use
■ For children who are younger than 3 months old. (new born baby)
■ If you ever had an allergic reaction to this product or any of its ingredients.
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or a pharmacist.
■ If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease) or 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or a pharmacist)
Questions or Comments?
1-562-695-2818 Monday - Friday 9AM-5PM WET
Distributed by: Bio Mission Group Inc.
Stop use and ask a doctor if
■ nervousness, dizziness, or sleeplessness, constipation occurs
■ redness or swelling is present ■ fever get worse or lasts more than 3 days
■ new severe symptoms occur such as shock (anaphylaxis), Stevens Johnson Syndrome, Lyell Syndrome, Asthma, Dyshepatia or Interstitial lung diseases.
■ pain, cough, nasal congestion gets worse or lasts after 5-6 times of taking a dose.
When using this product
■ Do not exceed recommended dose. Do not drink when taking doses
■ Avoid drive or use of machineries.
Other Information
■ Store between room temperature 20-25°C (68-77°F)
■ Avoid direct light or humid condition and excessive heat
Overdose warning
Do not exceed recommended dose. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-333-2333).
NDC 69365-003-01
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
Facol Cold & Flu Night
MULTI
Soft liquid-filled capsule
Guaifenesin-Expectorant
Acetaminophen-Fever/pain reliever
Phenylephrine HCl-Nasal Decongestant
Dextromethorphan HBr- Cough Suppressant
Diphenhydramine HCl-Antihistamine
sleep aid
10 capsules
JW Pharmaceuticals