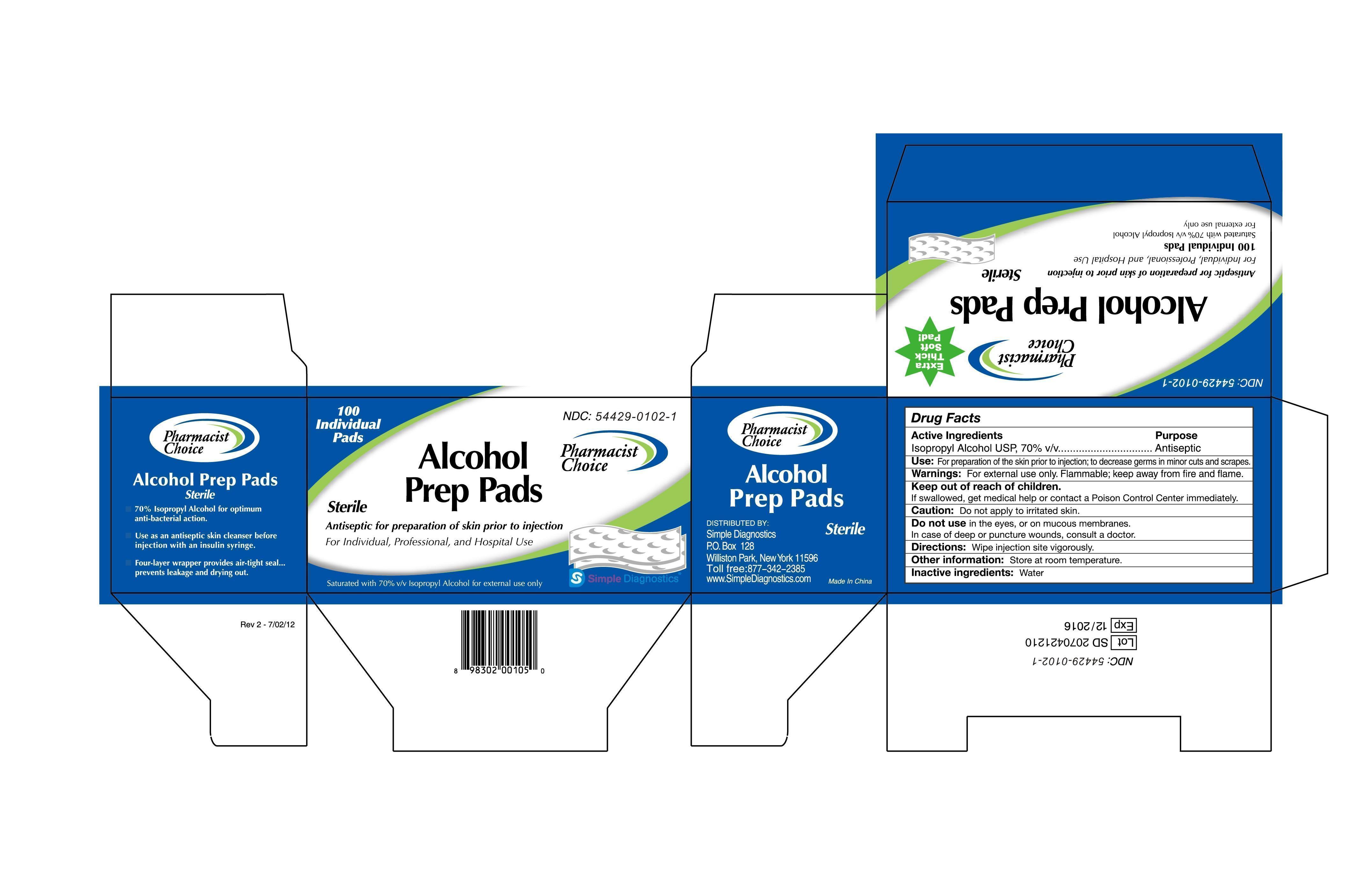
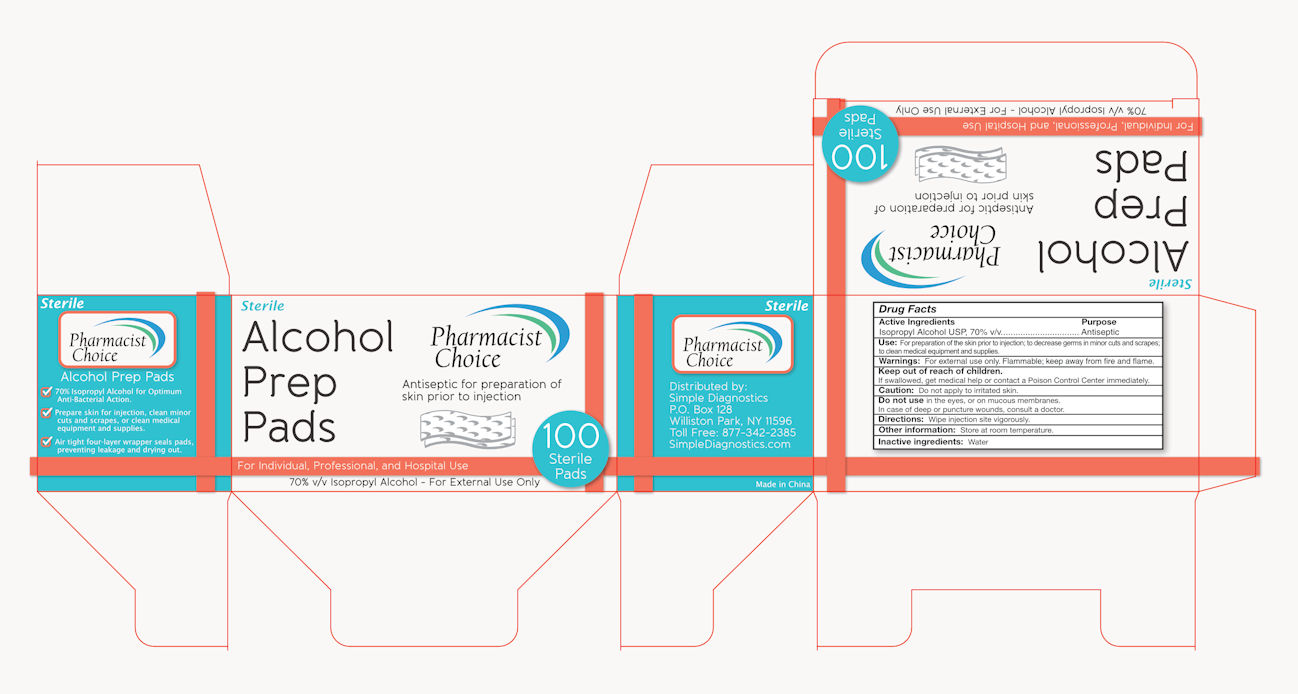
Drug Facts
Active Ingredients
Isopropyl Alcohol USP, 70% v/v
Purpose:
Antiseptic
Use: For preparation of skin prior to injection; to decrease germs in minor cuts and scrapes.
Warning: For external use only. Flammable, keep away from fire and flame.
Keep out of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Caution: Don’t apply to a irritated skin.
Don’t use in the eyes, or on mucous membranes.
In case of deep or puncture wounds, consult a doctor.
Directions: wipe injection site vigorously.
Other information: Store at room temperature.
Inactive ingredients: Water