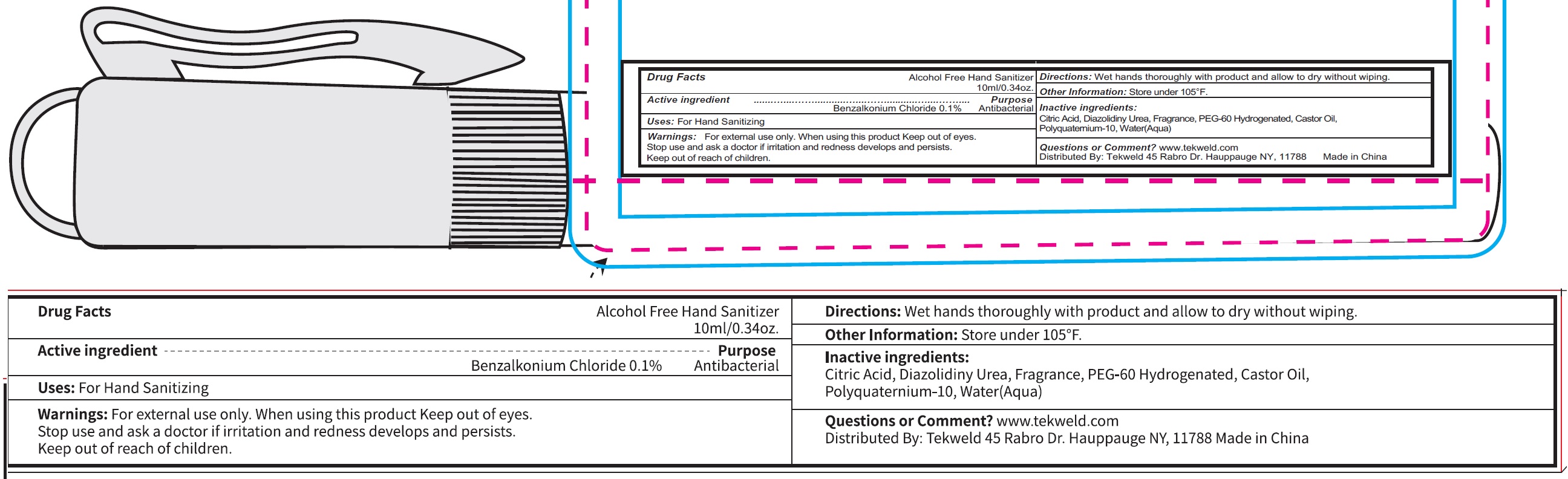
Active ingredient
Benzalkonium Chloride 0.1 %
Uses:
For Hand Sanitizing
Warnings:
For external use only.
When using this product
Keep out of eyes.
Stop use and ask doctor if
irritation and redness develops and persists.
Keep out of reach of children.
Directions:
Wet hands throughly with product and allow to dry without wiping.
Other Information:
Store under 105° F.
Inactive ingredients:
Citric Acid, Diazolidinyl Urea, Fragrance, PEG-60 Hydrogenated, Castor Oil, Polyquaternium-10,Water(Aqua)
Questions or Comment?
www.tekweld.com
Package Labeling:70412-120-10
Package labeling: 70412-120-30

Zhejiang Ayan Biotech Co.,Ltd.