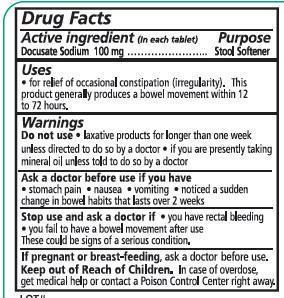
Uses
- for relief of occasional constipation (irregularity).This product generally produces a bowel movement within 12 to 72 hours.
Warnings
Do not use
- laxative products for longer than one week unless directed to do so by a doctor
- if you are presently taking mineral oil unless told to do so by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Directions
Adults and children 12 years of age and older: Take 1 tablet as needed, not to exceed more than 3 tablets daily, or as directed by a doctor.
Children under 12 years of age: Consult a doctor before use.
Other information
- Each tablet contains: Calcium 40 mg
- Each tablet contains: Sodium 10 mg
- Store at room temperature
- Do not use if imprinted safety seal is broken or missing
Inactive ingredients
Croscarmellose Sodium, Dicalcium Phosphate, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Pregelatinized Starch, Silica, Sodium Benzoate, Stearic Acid.