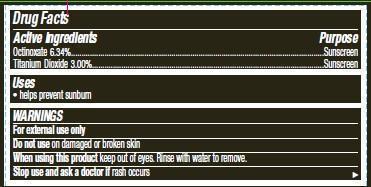
Active ingredients
Octinoxate 6.34%...................................
Titanium Dioxide 3.00%............................
Directions
• apply liberally 15 minutes before sun exposure
• children under 6 months of age: ask a doctor
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
Inactive ingredients: Propylene Glycol Dicaprylate/Dicaprate, Diisopropyl Dimer Dilinoleate, Microcrystalline Wax/Cire Microcristalline, Nylon-12, Cetyl Ricinoleate, Hydrogenated Coco-Glycerides, Silica, Tribehenin, Punica Granatum Sterols, Polymethyl Methacrylate, Ethylene/Methacrylate Copolymer, Propylparaben, VP/Hexadecene Copolymer, Dimethicone, Ethylparaben, Triethoxycaprylylsilane, Glyceryl Rosinate, Octyldodecyl Myristate, Magnesium Myristate, Butylparaben, Tocopheryl Acetate, Isopropyl Titanium Triisostearate. May Contain: Titanium Dioxide/CI 77891, Iron Oxides, Mica/CI 77019, Bismuth Oxychloride/CI 77163.