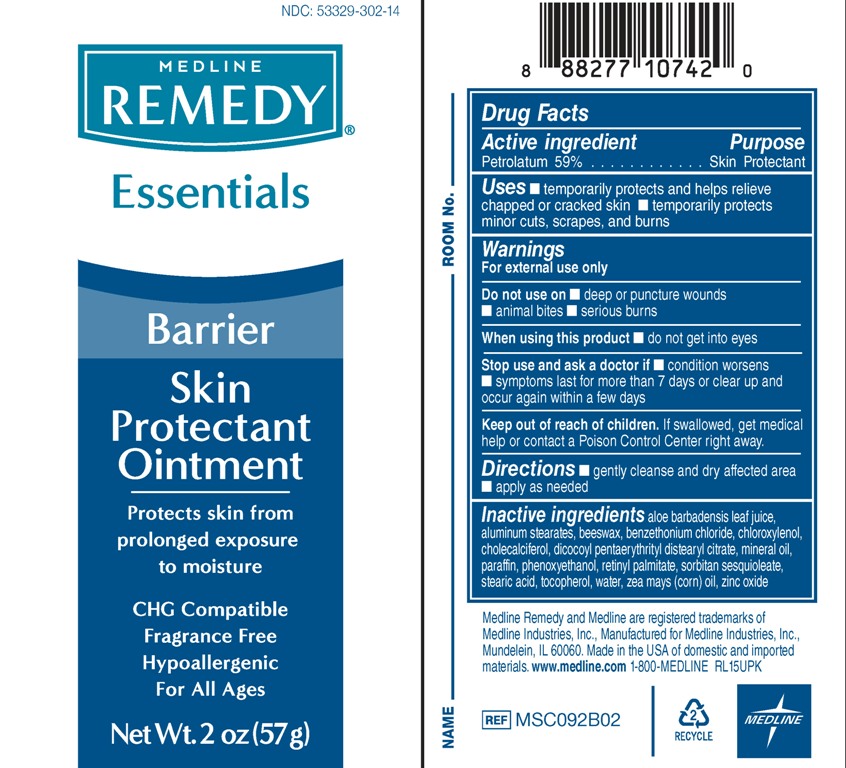
REMEDY SKIN PROTECTANT- white petrolatum paste
Medline Industries, LP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Petrolatum 59%
Uses
- Temporarily protects and helps relieve chapped or cracked skin
- Temporarily protects minor cuts, scrapes, and burns
Warnings
For external use only.
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens
- symptoms last for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- gently cleanse and dry affected area
- Apply as needed
Inactive ingredients
Aloe Barbadensis Leaf Juice, Aluminum Stearates, Beeswax, Benzethonium Chloride, Chloroxylenol, Cholecalciferol, Dicocoyl Pentaerythrityl Distearyl Citrate, Mineral Oil, Paraffin, Phenoxyethanol, Retinyl Palmitate, Sorbitan Sesquioleate, Stearic Acid, Tocopherol, Water, Zea Mays (Corn) Oil, Zinc Oxide
Package/Label Principal Display Panel
NDC 53329-302-14
Medline
REMEDY®
Essentials
Barrier Skin Protectant Ointment
Protects skin from prolonged exposure to moisture
CHG Compatible
FRAGRANCE FREE
Hypoallergenic
For All Ages
Net Wt. 2 oz (57 g)