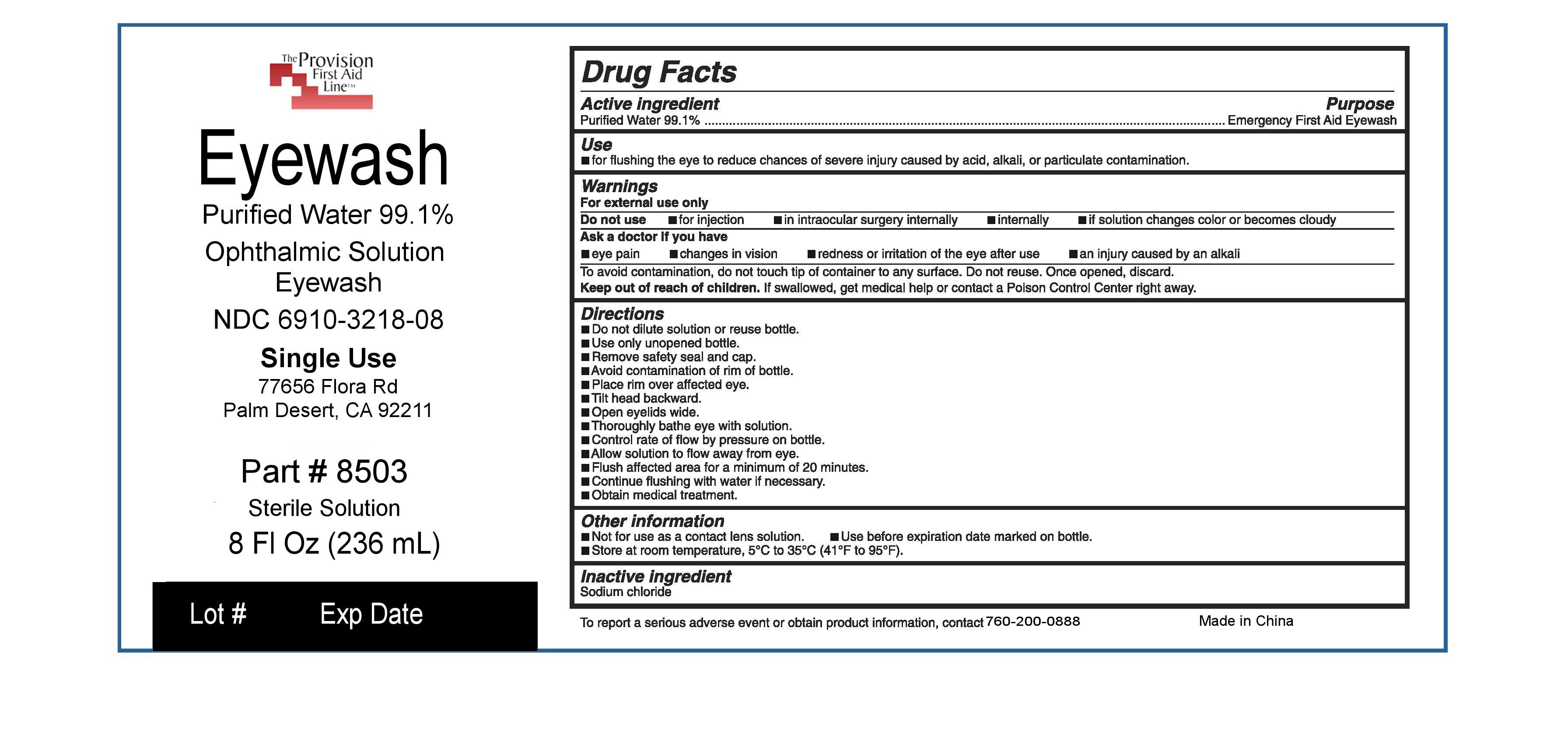
Uses:
fOR CLEANSING THE EYE TO HELP RELIEVE IRRITATION OR BURNING BY REMOVING LOOSE FOREIGN MATERIAL
KEEP OUT OF REACH OF CHILDREN. iF SWALLOWED, get medical help or
contact a Poison Control Center right
away.
Warnings:
For external use only.
Do not use if: you experience any open wounds in or near the eye and obtain immediate medical treatment.
If solution changes color or becomes cloudy.
When using this product -To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard
Stop use and ask a doctor if you have any of the following: Changes in vision. Eye pain. Condition worsens or persists. Continued redness or irritation of the eye.