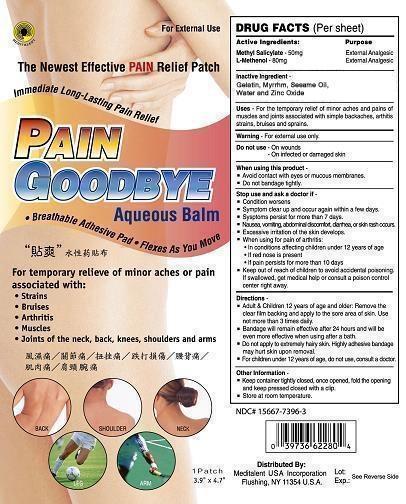
USES
Uses for the temporary relief of minor aches, and points of muscles and joints associated with simple backache, arthritis, strains, bruises and sprains.
Stop use and ask a doctor if
Keep out of reach of children to avoid accidental poisoning. If swallowed, get medical help or consult a poison control center right away.
Stop use and ask a doctor if
Condition worsens.
Symptom clears up and occurs again within a few days.
Symptom persists for more than 7 days.
Nausea, vomitting, abdominal discomfort, diarrhea, or skin rash occurs.
Excessive irritation of the skin develops.
Stop use and ask a doctor if
Avoid contact with eyes or mucous membranes.
Do not bandage tightly.
When using for pain of arthritis:
In conditions affecting children under 12 years of age
If red nose is present
If pain persists for more than 10 days
Directions
Adult and children 12 years of age and older; Remove the clear film backing and apply to the sore area of skin. Use not than 3 times daily.
Bandage will remain effective after 24 hours and will be even more effective when using after a bath.
Do not apply to extremely hairy skin. Highly adhesive bandage may hurt skin upon removal.
Other Information:
Keep container tightly closed. Once opened, fold the opening and keep pressed. Close with a clip.
Store at room temperature.