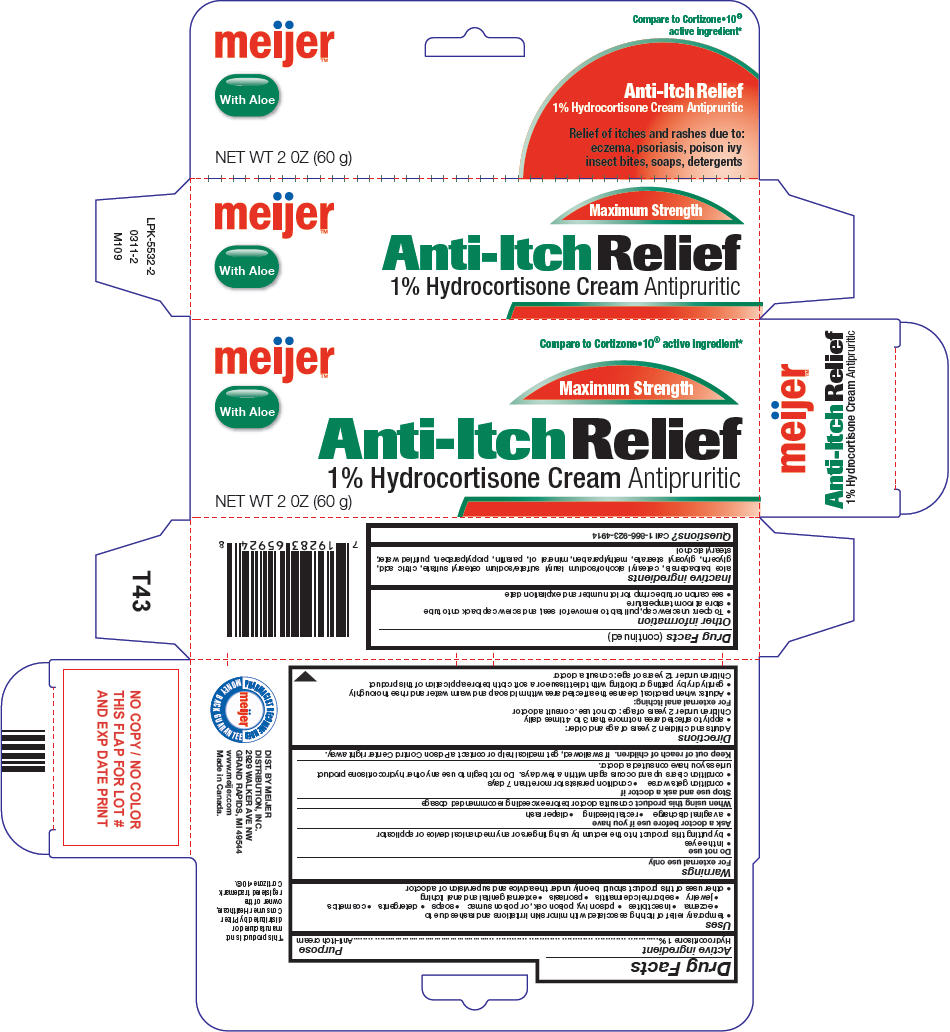
HYDROCORTISONE WITH ALOE- hydrocortisone cream
Meijer Distribution Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Hydrocortisone 1%
Uses
- temporary relief of itching associated with minor skin irritations and rashes due to
- eczema
- insect bites
- poison ivy, poison oak, or poison sumac
- soaps
- detergents
- cosmetics
- jewelry
- seborrheic dermatitis
- psoriasis
- external genital and anal itching
- other uses of this product should be only under the advice and supervision of a doctor
Warnings
For external use only
Do not use
- in the eyes
- by putting this product into the rectum by using fingers or any mechanical device or applicator
Ask a doctor before use if you have
- a vaginal discharge
- rectal bleeding
- diaper rash
When using this product consult a doctor before exceeding recommended dosage
Stop use and ask a doctor if
- condition gets worse
- condition persists for more than 7 days
- condition clears up and occurs again within a few days. Do not begin to use any other hydrocortisone product unless you have consulted a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 2 years of age and older:
- apply to affected area not more than 3 to 4 times daily
Children under 2 years of age: do not use. consult a doctor
For external anal itching:
- Adults: when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product
Children under 12 years of age: consult a doctor
Other information
- To open: unscrew cap, pull tab to remove foil seal, and screw cap back onto tube
- store at room temperature
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
aloe barbadensis, cetearyl alcohol/sodium lauryl sulfate/sodium cetearyl sulfate, citric acid, glycerin, glyceryl stearate, methylparaben, mineral oil, paraffin, propylparaben, purified water, stearyl alcohol
Questions?
Call 1-866-923-4914
DIST. BY MEIJER
DISTRIBUTION, INC.
2929 WALKER AVE NW
GRAND RAPIDS, MI 49544
PRINCIPAL DISPLAY PANEL - 60 g Tube Carton
meijer™
Compare to Cortizone•10® active ingredient*
Maximum Strength
With Aloe
Anti-Itch Relief
1% Hydrocortisone Cream Antipruritic
NET WT 2 0Z (60 g)