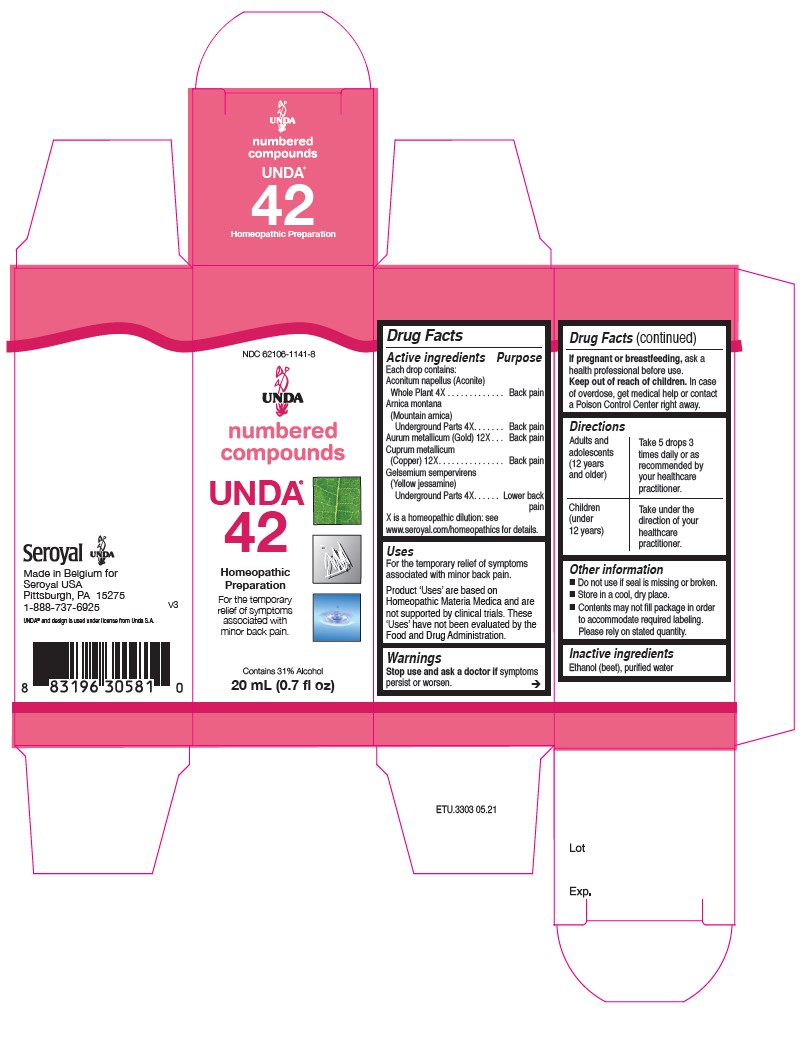
Active ingredients
Each drop contains equal parts of:
Aconitum napellus (Aconite) Whole Plant 4X
Arnica montana (Mountain arnica) Root 4X
Aurum metallicum (Gold) 12X
Cuprum metallicum (Copper) 12X
Gelsemium sempervirens (Yellow jessamine) Root 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a
Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Indications
For the relief of symptoms associated with minor back pain.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.