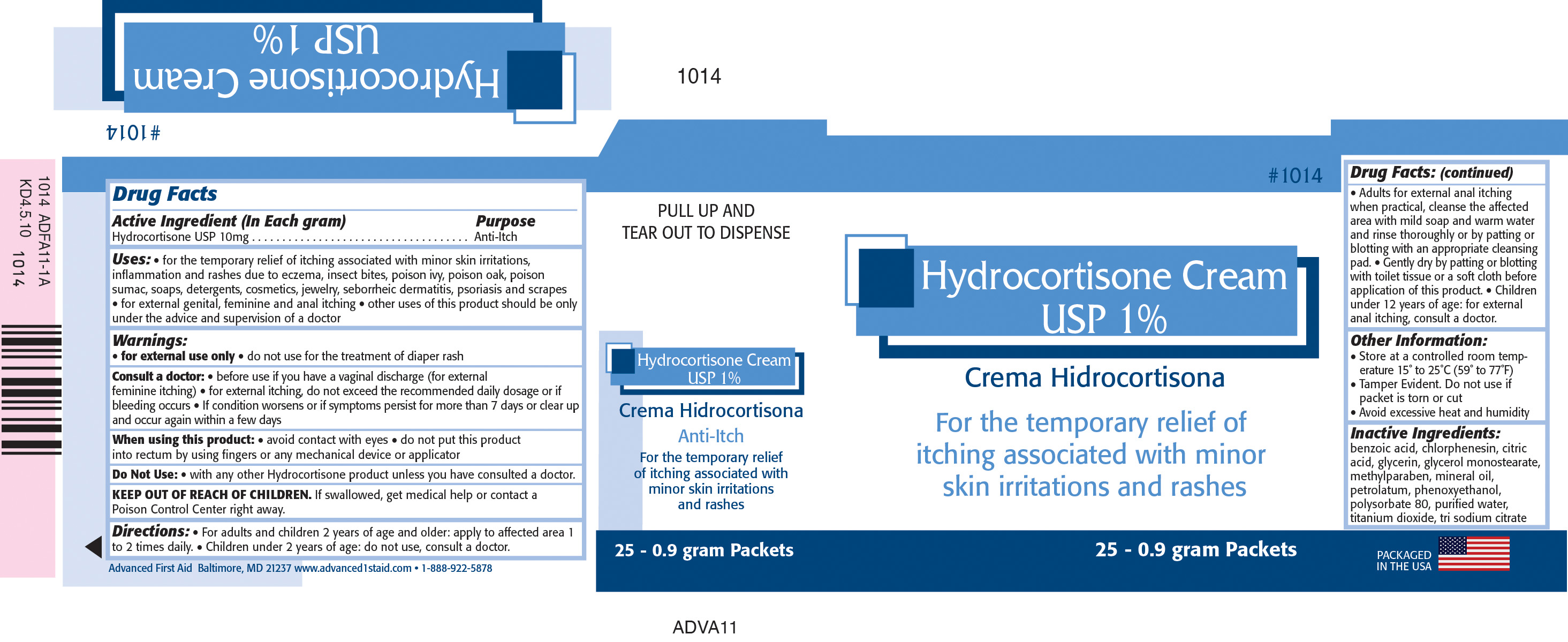
Uses:
• for the temporary relief of itching associated with minor skin irritations, inflammation and rashes due
to eczema, insect bites, poison ivy, poison oak, poison sumac, soaps, detergents, cosmetics, jewelry,
seborrheic dermatitis, psoriasis and scrapes
• for external genital, feminine and anal itching • other uses of this product should be only under the
advice and supervision of a doctor
Warnings:
• for external use only • do not use for the treatment of diaper rash
Consult a doctor: • before use if you have a vaginal discharge (for external feminine itching)
• for external itching, do not exceed the recommended daily dosage or if bleeding occurs
• If condition worsens or if symptoms persist for more than 7 days or clear up and occur again within
a few days
When using this product: • avoid contact with eyes • do not put this product into rectum by using
fingers or any mechanical device or applicator
Do Not Use: • with any other Hydrocortisone product unless you have consulted a doctor.
KEEP OUT OF REACH OF CHILDREN. If swallowed, get medical help or contact a Poison Control
Center right away.
Directions: • For adults and children 2 years of age and older: apply to affected area 1 to 2 times
daily. • Children under 2 years of age: do not use, consult a doctor. • Adults for external anal itching
when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly or by
patting or blotting with an appropriate cleansing pad. • Gently dry by patting or blotting with toilet tissue or a
soft cloth before application of this product. • Children under 12 years of age: for external anal itching,
consult a doctor.