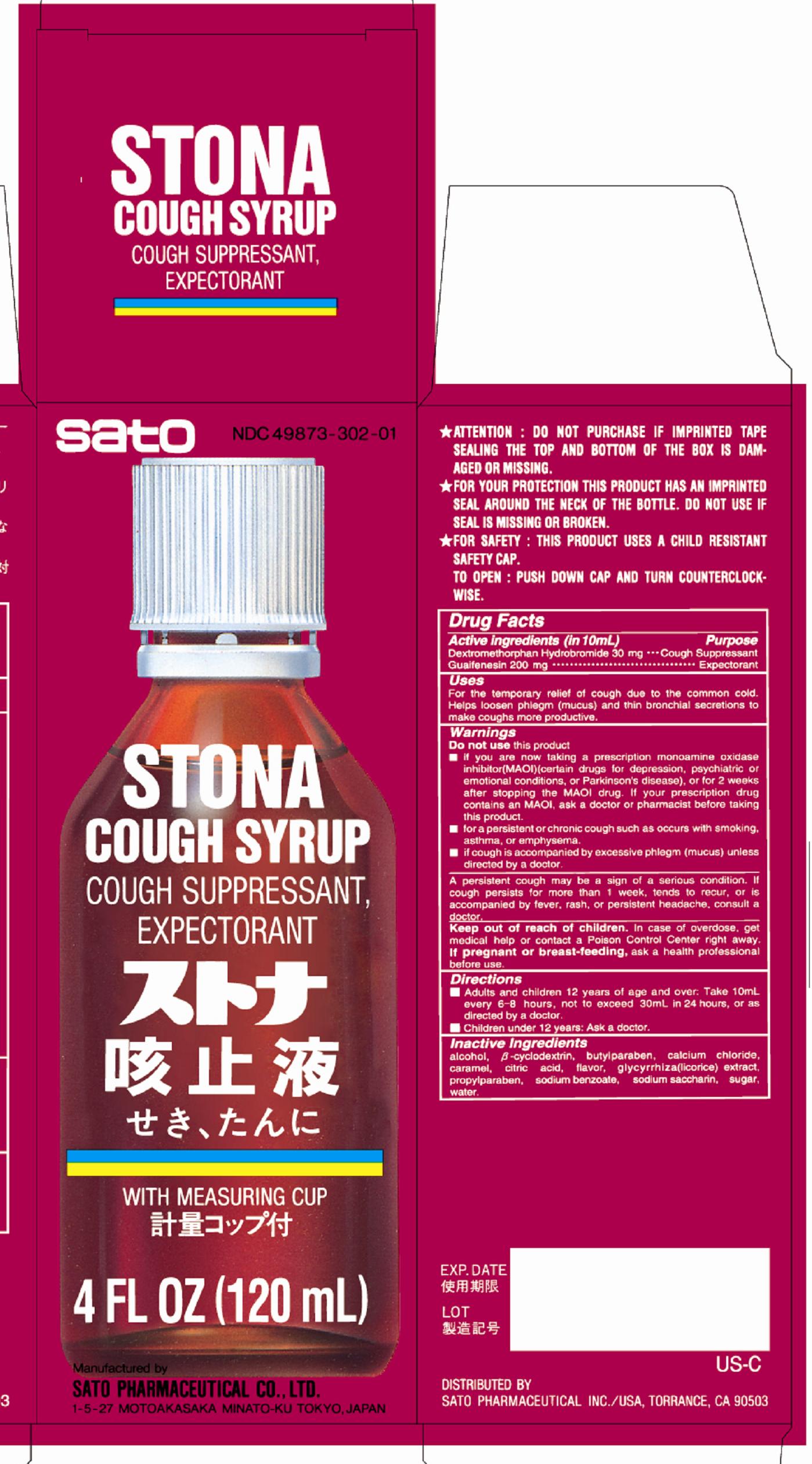
Uses
For the temporary relief of cough due to the common cold. Helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive.
WARNINGS
Enter section text here
Do not use this product
■if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
■for a persistent or chronic cough such as occurs with smoking, asthma, or emphysema.
■if cough is accompanied by excessive phlegm (mucos) unless directed by a doctor.
A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor.
Directions
■Adults and children 12 years of age and over: Take 10mL every 6 – 8 hours, not to exceed 30mL in 24 hours, or as directed by a doctor.
■Children under 12 years: Ask a doctor.