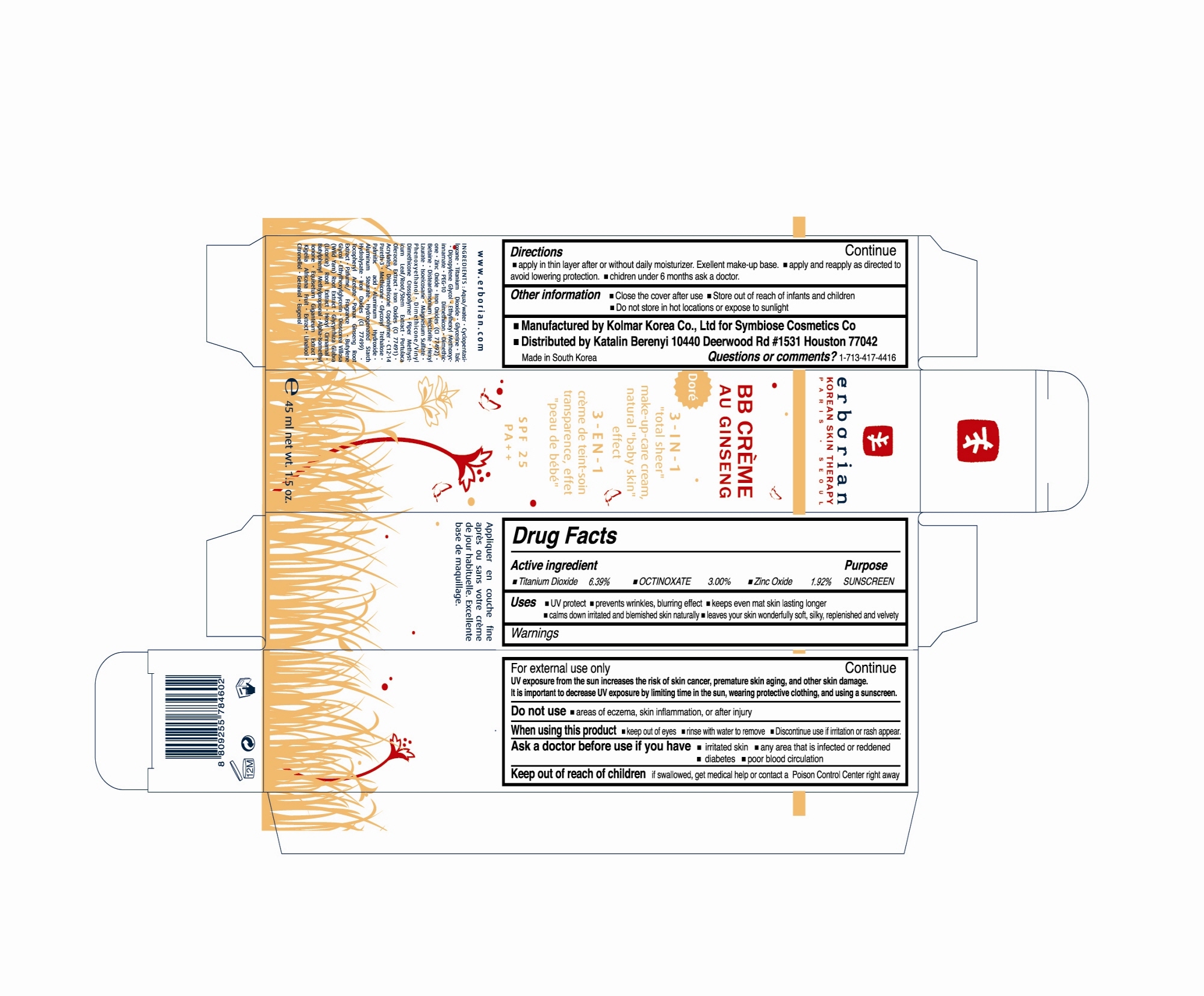
ERBORIAN BB CREME AU GINSENG DORE- titanium dioxide cream
Kolmar Korea Co Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Erborian BB Crème Au Ginseng Doré
Drug Facts
Active Ingredient
Titanium Dioxide 6.39%
Octinoxate 3.00%
Zinc Oxide 1.92%
Uses
Helps prevent sunburn. Provides High protect from sunburn. Higher SPF gives more sunburn protection.
Directions
Apply after basic skin care product. Spread and appropriate amount entire face following the skin texture. Reapply as required.
Warnings
For external use only.
UV exposure from the sun increases the risk of skin cancer, premature skin aging, and other skin damage. It is important to decrease UV exposure by limiting time in the sun, wearing protective clothing, and using a sunscreen.
When using the product Keep out of eyes. Rinse with water to remove.
Keep out of reach of children if swallowed, get medical help or contact a Poison Control Center
| ERBORIAN BB CREME AU GINSENG DORE
titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Kolmar Korea Co Ltd (688732723) |