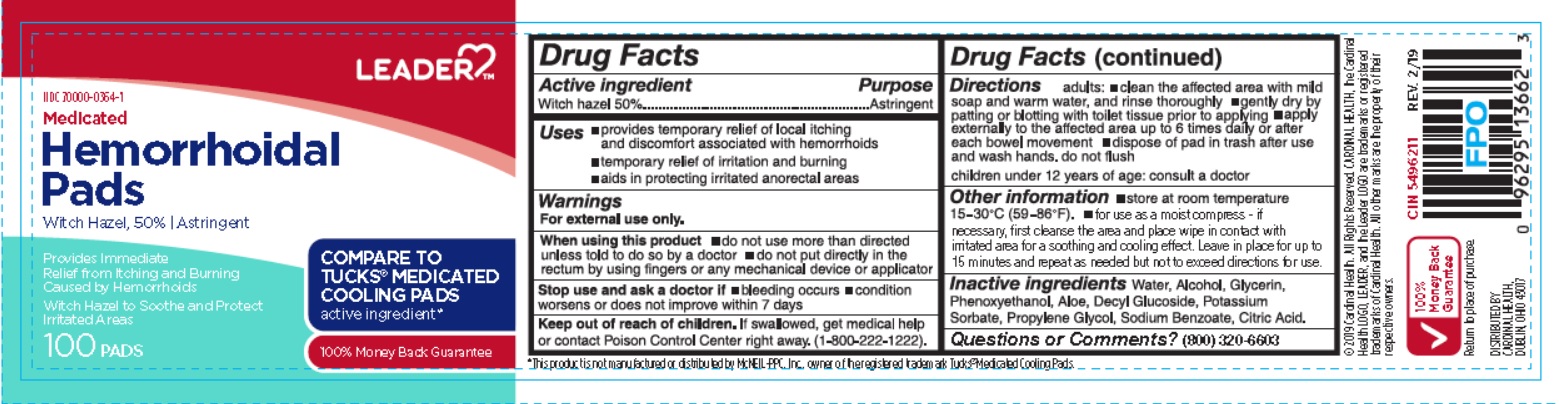
Uses
- provides temporary relief of local itching and discomfort associated with hemorrhoids
- temporary relief of irritation and burning.
- aids in protecting irritated anorectal areas.
Warnings
For external use only.
Directions
adults:
- cleane the affected area with mild soap and warm water, and rinse thoroughly
- gently dry by patting or blotting with toilet tissue prior to applying.
- apply externally to the affected area up to 6 times daily or after each bowel movement
- dispose of pad in thrash after use and wash hands, do not flush
children under 12 years of age: consult a doctor
Other Information
- store at room temperature 15- 30 oC (59 - 86 oF)
- for use as a moist compress - if necessary, first cleanse the area and place wipe in contact with irritated area for a soothing and cooling effect. Leave in place for up to 15 minutes and repeat as needed but not to exceed directions for use.
Inactive ingredients
Water, Alcohol, Glycerin, Phenoxyethanol, Aloe, Decyl Glucoside, Potassium Sorbate, Propylene Glycol, Sodium Benzoate, Citric Acid.
Principal Display Panel
LEADER TM
NDC 70000-0364-1
Medicated Hemorrhoidal Pads
WITCH HAZEL, 50% | Astringent
Provides Immediate Relief from Itching and Burning Caused by Hemorrhoids
Witch Hazel to Soothe and Protect Irritated Areas
COMPARE TO TUCKS® MEDICATED COOLING PADS active ingredient*
100% Money Back Guarantee
100 PADS
*This product is not manufactured or distributed by McNEIL-PPC, Inc., owner of the registered trademark Tucks® Medicated Cooling Pads.