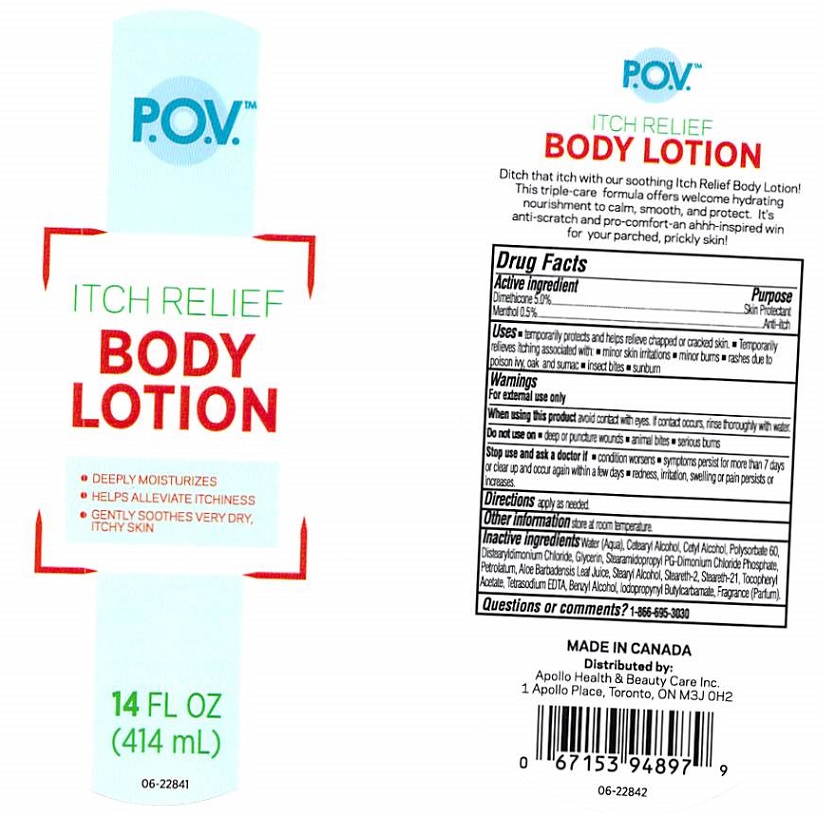
P.O.V ITCH RELIEF BODY- dimethicone, menthol lotion
Apollo Health and Beauty Care Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Dimethicone 5.0%, Menthol 0.5%
Purpose
Skin Protectant, Anti-itch
Uses
- temporarily protects and helps relieve chapped or cracked skin.
- temporarily relieves itching associated with: minor skin irritations, minor burns, rashes due to poison ivy, oak and sumac
- insect bites
- sunburn
Warnings
For external use only
When using this product
avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Do not use on
- deep or puncture wournds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness, irritation, swelling or pain persists or increases
Keep out of reach of children.
In case of accidental ingestion, get medical help or contact a Poison Control Center immediately.
Directions
apply as needed
Other information
store at room temperature.
Inactive ingredients
Water (Aqua), Cetearyl Alcohol, Cetyl Alcohol, Polysorbate 60, Distearyldimonium Chloride, Glycerin, Stearamidopropyl PG-Dimonium Chloride Phosphate, Petrolatum, Aloe Barbadensis Leaf Juice, Stearyl Alcohol, Steareth-2, Steareth-21, Tocopheryl Acetate, Tetrasodium EDTA, Benzyl Alcohol, Iodopropynyl Butylcarbamate, Fragrance (Parfum).
Questions or comments?
1-866-695-3030
Label Copy
Apollo Health and Beauty Care Inc.
