Uses
- First aid to help prevent the infection in minor cuts, scrapes and burns
- For the temporary relief of pain or itching.
Do not use
- in the eyes
- over large areas of the body
- if allergic to any of the ingredients
- longer than 1 week unless directed by a doctor.
Consult a doctor
- if under 2 years of age
- in case of deep cuts or puncture wounds, animal or human bites, or serious burns
- if pregnant or breast feeding.
Stop use and consult a doctor
- if the condition persists or worsens
- if symptoms persist for more than 7 days or clear up and occur again within a few days
- if a rash or other allergic reaction develops.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions for use
- Clean the affected area
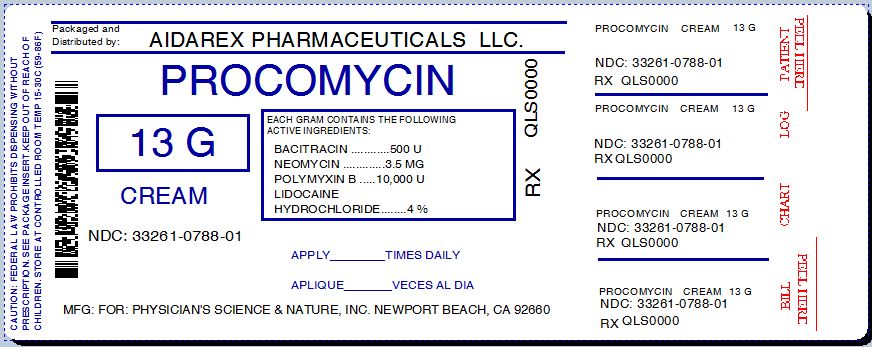
- apply a small amount of this product (an amount equal to the surface area of the tip of the finger) on the area 3 times daily.
- may be covered with a sterile bandage
- store at controlled room temperature 15°-30° (59°-86°).
Inactive ingredients
acrylates/dimethicone coploymer, allantoin, aloe barbadensis (aloe vera) leaf juice, arachidyl alcohol, arachidyl glucoside, arnica montana flower extract, ascorbic acid (vitamin C), behenyl alcohol, beta-glucan, benzalkonium chloride, beta-glucan, butyrospermum parkii (shea) butter, C 12-15 alkyl benzoate, caprylic/capric triglyceride, cetearyl alcohol, cetearyl glucoside, cyclopentasiloxane, dimethicone, dipalmitoyl hydroxyproline, glycerin, glycine soja (soybean) oil, helianthus annuus (sunflower) seed oil, hydrolyzed mytilus edulis byssus, methylparaben, panthenol, persea gratissima (avocado) oil, phenoxyethanol, polyglyceryl-6 distearate, propylparaben, sclerotium gum, tocopherol, water (aqua)
Manufactured in the USA for:
Physician's Science and Nature, Inc.
220 Newport Center Drive, 11-634,
Newport Beach, CA 92660
Repackaged By:
Aidarex Pharmaceuticals, LLC.
Corona, CA 92880