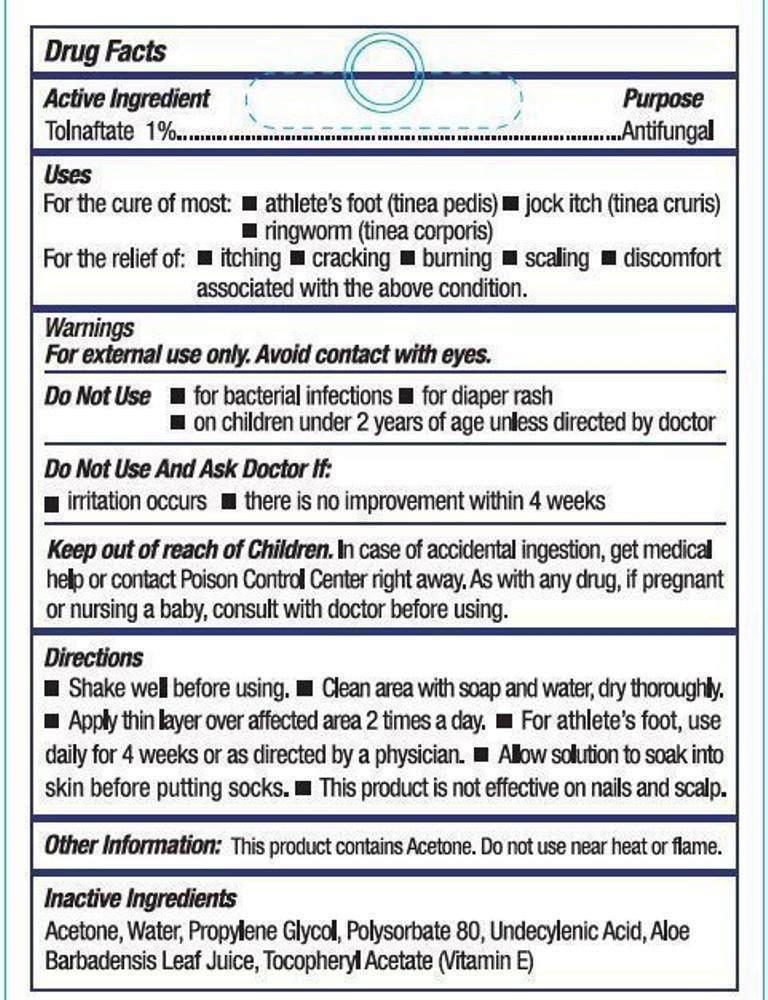
Uses
For the cure of:
- athlete's foot (tinea pedis)
- jock itch (tinea cruris)
- ringworm (tinea corporis)
For the relief of:
- itching
- cracking
- burning
- scaling
- discomfort associated with the above condition
Do not use
- for bacterial infections
- for diaper rash
- on children under 2 years of age unless directed by a doctor
KEEP OUT OF REACH OF CHILDREN.
In case of accidental ingestion, get medical help or contact Poison
Control Center right away. As with any drug, if pregnant or nursing a baby, consult with a doctor before using.
Directions
- Shake well before using
- Clean area with soap and water, dry thoroughly
- Apply thin layer over affected area 2 times a day
- For athlete's foot, use daily for 4 weeks or as directed by a physician
- Allow solution to soak into the skin before putting socks on
- This product is not effective on nails and scalp
 FungiGoneHalfOunceCarton.jpg
FungiGoneHalfOunceCarton.jpg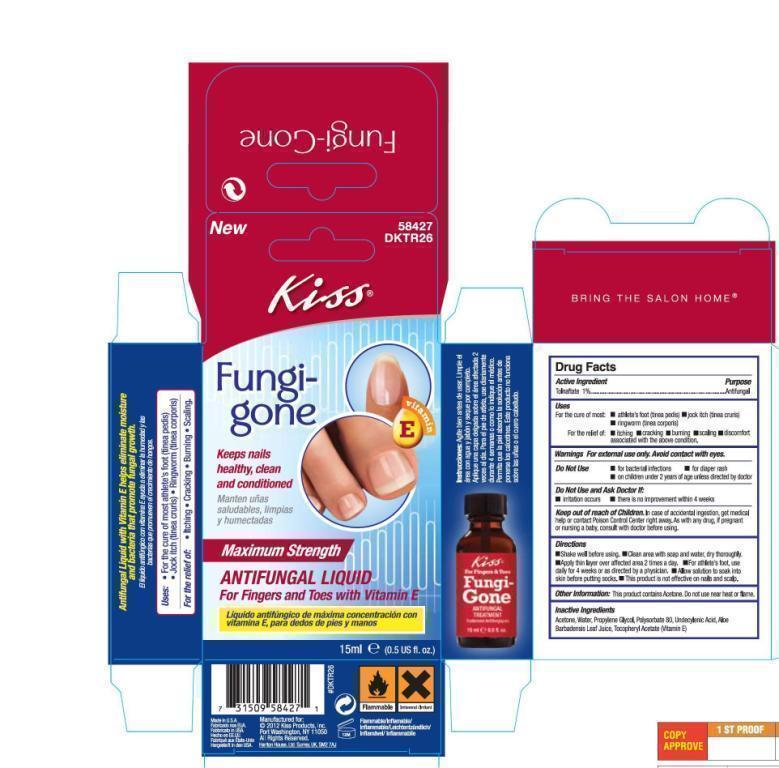 FungiGoneOneOunceLabel.jpg
FungiGoneOneOunceLabel.jpg FungiGoneOneOunceCarton1.jpg
FungiGoneOneOunceCarton1.jpg FungiGoneOneOunceCarton2.jpg
FungiGoneOneOunceCarton2.jpg