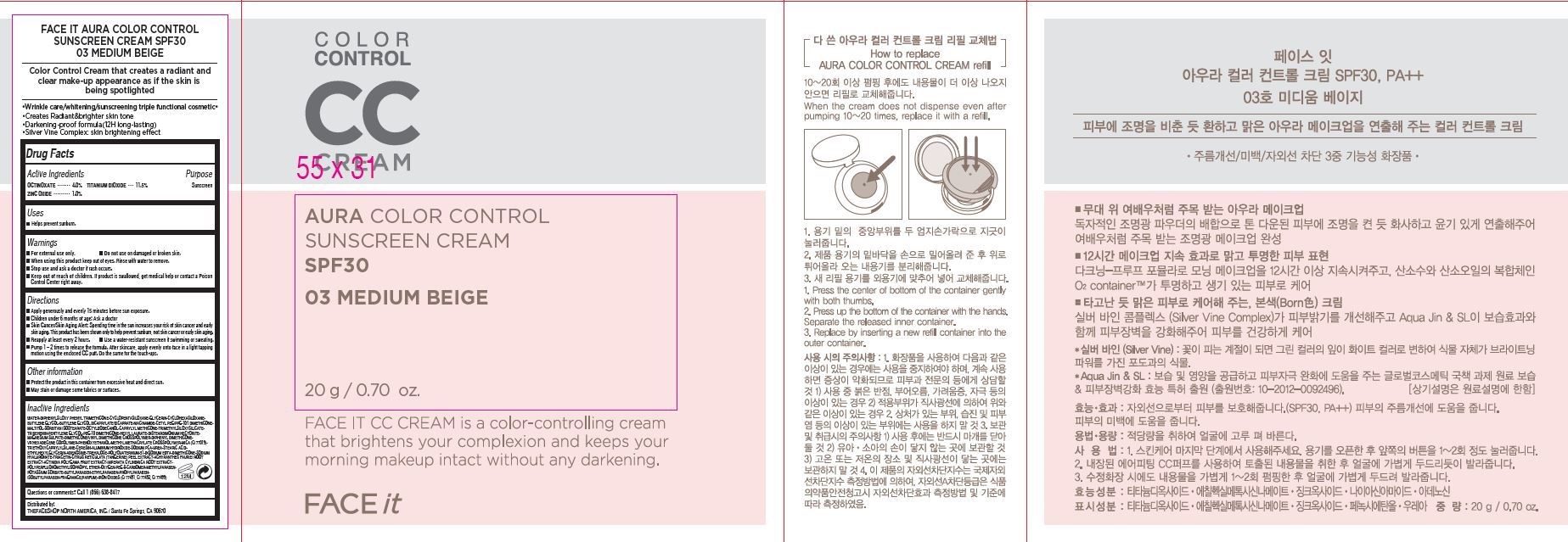
Active Ingredients
OCTINOXATE.............. 4.0%
TITANIUM DIOXIDE. 11.5%
ZINC OXIDE................. 1.0%
Warnings
For external use only.
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply generously and evenly 15 minutes before sun exposure.
Children under 6 months of age: Ask a doctor
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin. aging. This product has been shown only to help prevent sunburn,
not skin cancer or early skin aging.
Reapply at least every 2 hours
Use a water-resistant sunscreen if swimming or sweating.
1. Pump 1 – 2 times to release the formula.
2. After skin care, apply evenly onto face in a light tapping motion using the enclosed CC puff.
3. Do the same for the touch-ups
Other Information
Protect the product in this container from excessive heat and direct sun
May stain or damage some fabrics or surfaces
Inactive Ingredients
WATER•DIPHENYLSILOXY PHENYL TRIMETHICONE•CYCLOPENTASILOXANE•GLYCERIN•CYCLOHEXASILOXANE•
BUTYLENE GLYCOL•BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE•NIACINAMIDE•CETYL PEG/PPG-10/1 DIMETHICONE•
MALTITOL•SORBITAN ISOSTEARATE•OCTYLDODECANOL•CAPRYLYL METHICONE•TRIMETHYLSILOXYSILICATE•
TRIBEHENIN•PENTYLENE GLYCOL•PEG-10 DIMETHICONE•HEXYL LAURATE•DISTEARDIMONIUM HECTORITE•
MAGNESIUM SULFATE•DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER•DIPHENYL DIMETHICONE•
VP/HEXADECENE COPOLYMER•PHENOXYETHANOL•METHYL METHACRYLATE CROSSPOLYMER•MICA (CI 77019)•
TRIETHOXYCAPRYLYLSILANE•CERESIN•ALUMINUM HYDROXIDE•SODIUM PCA•UREA•STEARIC ACID•
ETHYLHEXYLGLYCERIN•ADENOSINE•TREHALOSE•POLYQUATERNIUM-51•DISODIUM EDTA•DIMETHICONE•SODIUM
HYALURONATE•TRIACETIN•CITRUS RETICULATA (TANGERINE) PEEL EXTRACT•ACHYRANTHES FAURIEI ROOT
EXTRACT•ACTINIDIA POLYGAMA FRUIT EXTRACT•IMPERATA CYLINDRICA ROOT EXTRACT•
POLYPERFLUOROMETHYLISOPROPYL ETHER•OXYGEN•PEG-8•CARBOMER•METHYLPARABEN•
POTASSIUM SORBATE•BUTYLPARABEN•ETHYLPARABEN•PROPYLPARABEN•
ISOBUTYLPARABEN•FRAGRANCE(PARFUM)•IRON OXIDES (CI 77491, CI 77492, CI 77499)