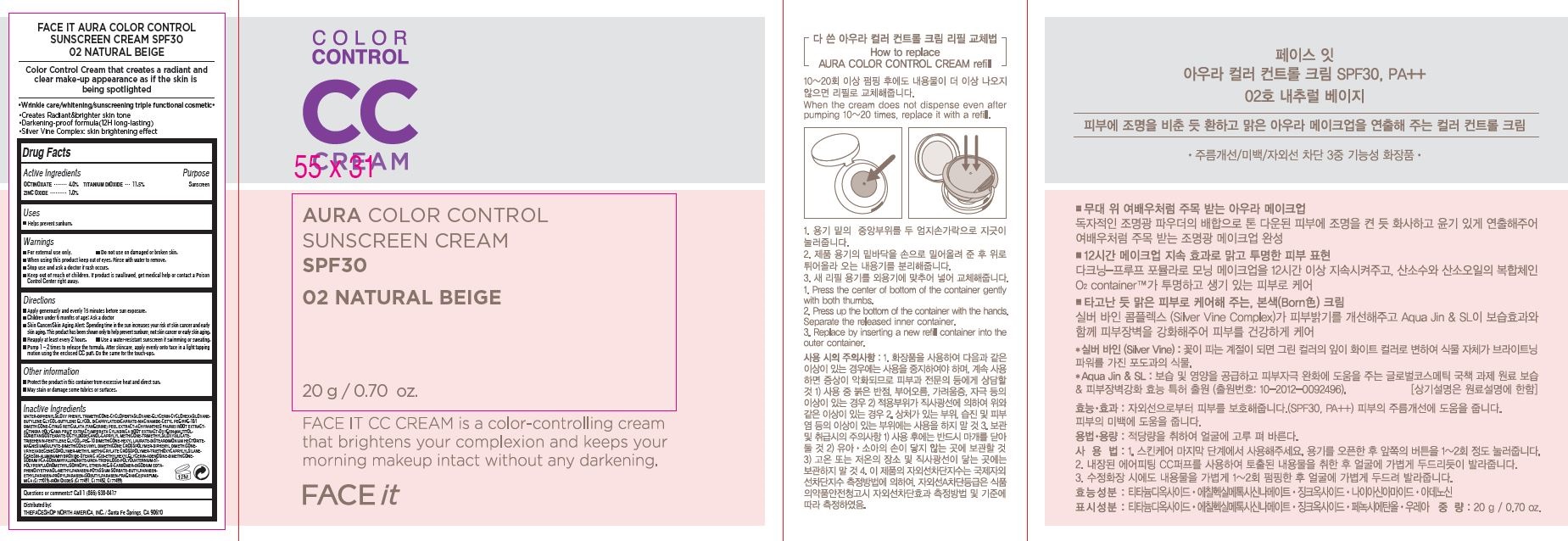
Active Ingredients
OCTINOXATE............ 4.0%
TITANIUM DIOXIDE 11.5%
ZINC OXIDE................ 1.0%
Warnings
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison
Control Center right away.
Directions
Apply generously and evenly 15 minutes before sun exposure.
Children under 6 months of age: Ask a doctor
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early
skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
Reapply at least every 2 hours. Use a water-resistant sunscreen if swimming or sweating.
Pump 1 – 2 times to release the formula. After skincare, apply evenly onto face in a light tapping
motion using the enclosed CC puff. Do the same for the touch-ups.
Other information
Protect the product in this container from excessive heat and direct sun.
May stain or damage some fabrics or surfaces.
Inactive Ingredients
WATER•DIPHENYLSILOXY PHENYL TRIMETHICONE•CYCLOPENTASILOXANE•GLYCERIN•CYCLOHEXASILOXANE•
BUTYLENE GLYCOL•BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE•NIACINAMIDE•CETYL PEG/PPG-10/1
DIMETHICONE•CITRUS RETICULATA (TANGERINE) PEEL EXTRACT•ACHYRANTHES FAURIEI ROOT EXTRACT•
ACTINIDIA POLYGAMA FRUIT EXTRACT•IMPERATA CYLINDRICA ROOT EXTRACT•OXYGEN•MALTITOL•
SORBITANISOSTEARATE•OCTYLDODECANOL•CAPRYLYL METHICONE•TRIMETHYLSILOXYSILICATE•
TRIBEHENIN•PENTYLENE GLYCOL•PEG-10 DIMETHICONE•HEXYL LAURATE•DISTEARDIMONIUM HECTORITE•
MAGNESIUMSULFATE•DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER•DIPHENYL DIMETHICONE•
VP/HEXADECENECOPOLYMER•METHYL METHACRYLATE CROSSPOLYMER•TRIETHOXYCAPRYLYLSILANE•
CERESIN•ALUMINUMHYDROXIDE•STEARIC ACID•ETHYLHEXYLGLYCERIN•ADENOSINE•DIMETHICONE•
SODIUM PCA•SODIUMHYALURONATE•UREA•TREHALOSE•POLYQUATERNIUM-51•
POLYPERFLUOROMETHYLISOPROPYL ETHER•PEG-8•CARBOMER•DISODIUM EDTA•
PHENOXYETHANOL•METHYLPARABEN•POTASSIUM SORBATE•BUTYLPARABEN•
ETHYLPARABEN•PROPYLPARABEN•ISOBUTYLPARABEN•FRAGRANCE(PARFUM)•
MICA (CI 77019)•IRON OXIDES (CI 77491, CI 77492, CI 77499)