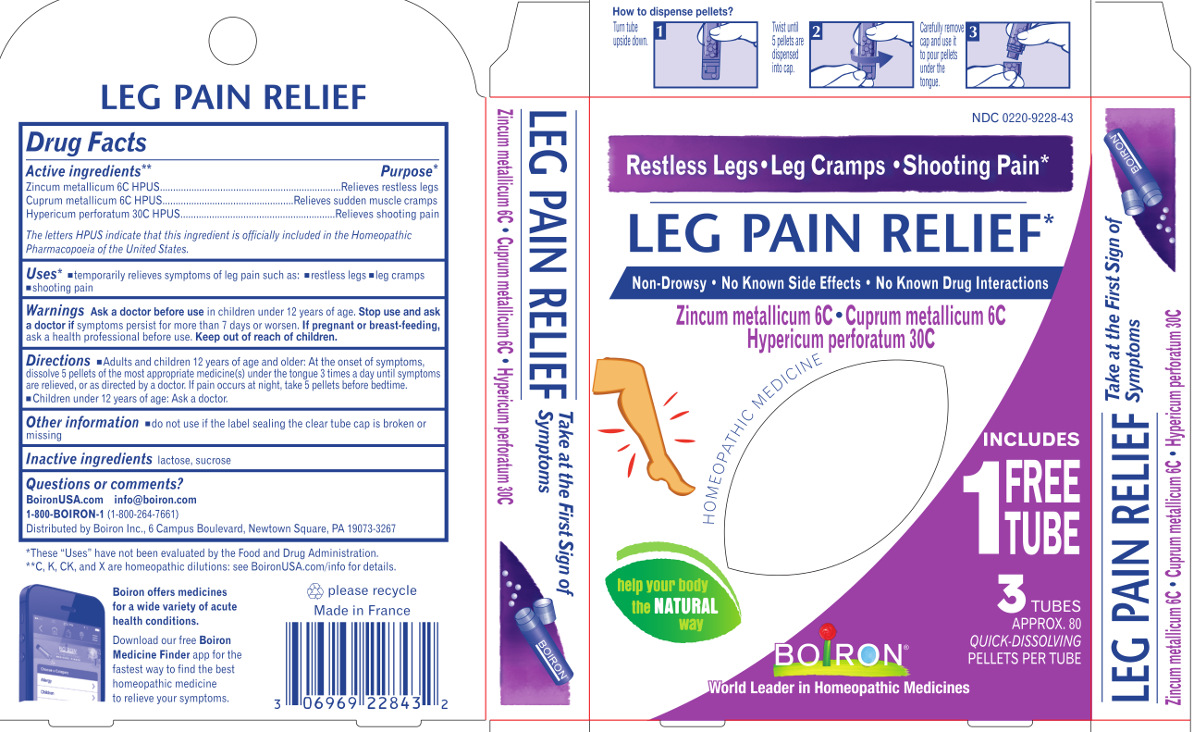
Active Ingredients**
Zincum metallicum 6C HPUS
Cuprum metallicum 6C HPUS
Hypericum perforatum 30C HPUS
The letters HPUS indicate that this ingredient is officially included
in the Homeopathic Pharmacopoeia of the United States.
Purpose*
Zincum metallicum 6C HPUS ..... Relieves restless legs
Cuprum metallicum 6C HPUS ..... Relieves sudden muscle cramps
Hypericum perforatum 30C HPUS ..... Relieves shooting pain
Directions
Adults and children 2 years of age and older:
At the onset of symptoms, dissolve 5 pellets of the most appropriate medicine(s)
under the tounge 3 times a day until symptoms are releived, or as directed by a doctor.
If pain occurs at night, take 5 pellets before bedtime.
Children under 2 years of age: Ask a doctor.
Questions, Comments?
BoironUSA.com
info@boiron.com
1-800-BOIRON-1 (1-800-264-7661)
Distributed by Boiron Inc., 6 Campus Boulevard,
Newtown Square, PA 19073-3267